4-氟托咪酯(NH600001)在健康受试者中的安全性、耐受性和药代动力学:一项首次人体随机对照I期研究
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
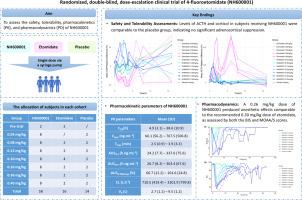
背景:NH600001(原EL-0052)是一种新型的4-氟咪酯脂质乳剂。这项首次人体、随机、双盲、安慰剂对照、单次递增剂量试验旨在评估NH600001的安全性、耐受性、药代动力学(PK)和药效学(PD)。方法:88名健康志愿者随机分为7个剂量组(0.04 ~ 0.40 mg/kg)。每个队列包括NH600001、安慰剂和依托咪酯作为有效比较剂。可注射乳剂作为一个单一的,10毫升静脉注射剂量超过一分钟使用注射泵。安全性通过临床评估、实验室检查、心电图和治疗不良事件(teae)来评估。测定血浆药物浓度进行PK分析,以双谱指数(BIS)、修正观察者警觉/镇静评价(MOAA/S)和睫毛反射作为PD指标。结果:88名参与者中,58人接受了NH600001, 16人接受了依托咪酯,14人接受了安慰剂。57例(64.8%)报告130例teae,多数为轻度;无严重不良事件报告。NH600001组TEAE发生率为62.1%,依托咪酯组为81.2%,安慰剂组为57.1%。与依托咪酯相比,NH600001不诱导肾上腺皮质抑制。各剂量组达到最大血药浓度的平均时间(Tmax)为2.5±0.9 ~ 3.9±3.3分钟,平均终末半衰期(t1/2)为4.9 ~ 38.6小时。浓度-时间曲线下的面积从0到无穷远(AUC0-∞),到0.04-0.40 mg/kg范围内的最后可量化浓度(AUC0-t),呈线性剂量-暴露关系。在0.26 mg/kg时,NH600001产生的麻醉起效(1.8分钟)和持续时间(4.9分钟)与0.30 mg/kg依托咪酯相当。结论:与依托咪酯相比,NH600001表现出良好的安全性和耐受性,肾上腺皮质抑制降低。支持0.20-0.30 mg/kg的剂量用于进一步的临床开发。这些发现表明,NH600001可能是一种有希望的替代依托咪酯的方法,可以在保持麻醉效果的同时最大限度地减少内分泌不良反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Safety, tolerability, and pharmacokinetics of 4-fluoroetomidate (NH600001) in healthy subjects: a first-in-human, randomised, controlled, phase I study
Background
NH600001 (formerly EL-0052) is a novel 4-fluoroetomidate formulated as a lipid emulsion. This first-in-human, randomized, double-blind, placebo-controlled, single-ascending-dose trial aimed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of NH600001.
Methods
Eighty-eight healthy volunteers were randomized into seven dose cohorts (0.04–0.40 mg/kg). Each cohort included NH600001, placebo, and etomidate as an active comparator. The injectable emulsion was administered as a single, 10 mL intravenous dose over one minute using a syringe pump. Safety was assessed by clinical evaluations, laboratory tests, electrocardiography, and treatment-emergent adverse events (TEAEs). Plasma drug concentrations were measured for PK analysis, and bispectral index (BIS), modified observer assessment of alertness/sedation (MOAA/S), and eyelash reflex were used as PD indicators.
Results
Of 88 participants, 58 received NH600001, 16 received etomidate, and 14 received placebo. A total of 57 subjects (64.8 %) reported 130 TEAEs, most of which were mild; no serious adverse events were reported. TEAE incidence was 62.1 % in the NH600001 group, 81.3 % in the etomidate group, and 57.1 % in the placebo group. NH600001 did not induce adrenocortical suppression, in contrast to etomidate. Across the dose groups, the mean time to maximum plasma concentration (Tmax) ranged from 2.5 ± 0.9 to 3.9 ± 3.3 min, while the mean terminal half-life (t1/2) ranged from 4.9 to 38.6 h. A linear dose–exposure relationship was observed for area under the concentration–time curve from zero to infinity (AUC0–∞) and to the last quantifiable concentration (AUC0–t) across 0.04–0.40 mg/kg. At 0.26 mg/kg, NH600001 produced anesthetic onset (1.8 min) and duration (4.9 min) comparable to 0.30 mg/kg etomidate.
Conclusions
NH600001 demonstrated favorable safety and tolerability, with reduced adrenocortical suppression compared to etomidate. Doses of 0.20–0.30 mg/kg are supported for further clinical development. These findings suggest NH600001 may offer a promising alternative to etomidate by preserving anesthetic efficacy while minimizing endocrine adverse effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


