2-官能化(环)烷基-4.5-未取代恶唑合成中脱氮三唑环裂解
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
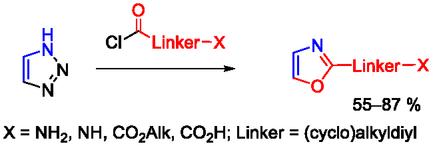
脱氮三唑环裂解应用于制备广泛的2-(环)烷基新唑衍生物。所建议的方法包括在亚砜中或在整洁的条件下进行1,2,3-三唑与各种功能取代的酸氯化物的反应。尽管该反应是放热性质的,但它提供了高收率的目标产物。在(环)烷基链上可以耐受各种官能团,如受保护的伯胺和仲胺和酯。所开发的方法用于制备具有恶唑基团的光学活性氨基酸。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Denitrogenative Triazole Ring Cleavage in Synthesis of 2‐Functionalized (Cyclo)alkyl‐4.5‐Unsubstituted Oxazoles
Denitrogenative triazole ring cleavage is applied for the preparation of a broad set of 2‐(cyclo)alkylo‐xazole derivatives. The proposed procedures involve carrying out the reaction of 1,2,3‐triazole with various functionally substituted acid chlorides in sulfolane or under neat conditions. Despite the exothermic nature of the reaction, it affords the target products in high yield. Various functional groups are tolerated in the (cyclo)alkyl chain such as protected primary and secondary amine and esters. The developed procedure is applied for the preparation of optically active amino acids featuring oxazole moiety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


