六氰钒阴极中质子协同铜离子存储实现了高性能水铜金属电池
IF 8.9
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
摘要
六氰高铁钒(VHCF)具有成本低、易于合成、容量大等优点,在多价离子电池正极材料中具有很大的应用潜力。但其在铜金属水溶液电池(acmb)中的电化学性能和机理尚未得到深入研究。本文采用纳米级VHCF作为acmb的正极材料,系统研究了在电解液中添加H2SO4对电池电化学性能的影响。VHCF||CuSO4 + H2SO4||Cu电池比VHCF||CuSO4||Cu电池具有更高的能量密度、功率密度和循环稳定性。其中,在0.2 A g−1时,放电容量可达81.6 mAh g−1,能量密度为55.6 Wh Kg−1;循环2000次后,比容量保持在48.7 mAh g−1,容量保持率为63.8%;电流密度为5 a g−1时,功率密度可达4213 W Kg−1。这种增强可归因于VHCF阴极中质子协同铜离子存储和反应动力学的增强,以及铜阳极的可逆性的提高。本研究为acmb电极材料和电解质的设计提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
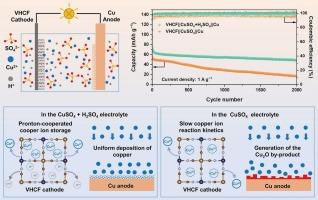
Proton-cooperated copper-ion storage in vanadium hexacyanoferrate cathode enables high-performance aqueous copper metal battery
Vanadium hexacyanoferrate (VHCF) has shown great application potential as a cathode material for multivalent-ion batteries due to its low cost, ease of synthesis, and high capacity. However, its electrochemical performance and mechanism in aqueous copper metal batteries (ACMBs) have not been explored. Herein, a nanosized VHCF was used as the cathode material for ACMBs, and the effects of adding H2SO4 to the electrolyte on the battery's electrochemical properties were systematically investigated. The VHCF||CuSO4 + H2SO4||Cu battery exhibited higher energy density, power density, and cycling stability than the VHCF||CuSO4||Cu battery. Specifically, the discharge capacity could reach 81.6 mAh g−1 with an energy density of 55.6 Wh Kg−1 at 0.2 A g−1; the specific capacity remained at 48.7 mAh g−1 after 2000 cycles with a capacity retention ratio of 63.8 %; the power density could reach 4213 W Kg−1 at a current density of 5 A g−1. This enhancement can be attributed to enhanced proton-cooperated copper ion storage and reaction kinetics in the VHCF cathode, along with improved reversibility of the copper anode. This work can offer new ideas for the design of electrode materials and electrolytes for ACMBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of energy storage
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
11.80
自引率
24.50%
发文量
2262
审稿时长
69 days
期刊介绍:
Journal of energy storage focusses on all aspects of energy storage, in particular systems integration, electric grid integration, modelling and analysis, novel energy storage technologies, sizing and management strategies, business models for operation of storage systems and energy storage developments worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


