负载型金属催化剂上Ru4/Cr2O3的CO2甲烷化机理及选择性加氢因素的DFT研究
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
揭示反应机制和确定选择性的驱动因素仍然是多相催化的巨大挑战。本文通过密度泛函理论计算,研究了缺氧Cr2O3(012)支持的Ru4簇(Ru4/Cr2O3 - ov)上的CO2甲烷化反应,并解析了M4/Cr2O3 - ov (M = Ru, Ni, Pt)表面产物选择性的来源。CO2吸附在桥式Ru位点上,容易被活化,而H₂在Ru位点上自发离解。CO2*→CO*→HCO*→CH2O*→CH2*→CH4的CO*途径是最有利的途径,其中CO2*的解离和CH2O*的脱氧是决定速率的步骤,能垒约为1.0 eV。对比分析表明,M4/ Cr2O3-Ov对氧的吸附能决定了CO2加氢选择性。此外,金属的功函数更小,加强了O*结合,从而促进了CH4的选择性。反之,功函数越大,O*吸附减弱,有利于CO生成。这些发现建立了金属的功函数作为调节CO2加氢选择性的内在的、实验上可获得的描述符,指导了负载金属催化剂的合理设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
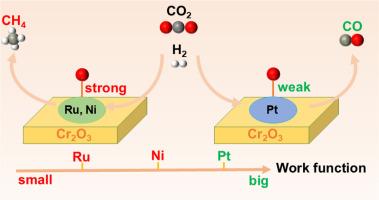
CO2 methanation mechanism on Ru4/Cr2O3 and factors for selective hydrogenation on supported metal catalysts: A DFT study
Unraveling reaction mechanisms and identifying the drivers of selectivity remain grand challenges in heterogeneous catalysis. Here, density functional theory calculations were performed to investigate CO2 methanation on an oxygen-deficient Cr2O3 (012)-supported Ru4 cluster (Ru4/Cr2O3–Ov) and to decode the origin of product selectivity across M4/Cr2O3–Ov (M = Ru, Ni, Pt) surfaces. CO2 adsorbs at bridge Ru sites and is readily activated, while H₂ dissociates spontaneously on Ru sites. The CO* pathway that CO2* → CO* → HCO* → CH2O* → CH2* → CH4, is the most favorable route, with the dissociation of CO2* and deoxygenation of CH2O* acting as rate-determining steps with energy barriers of approximately 1.0 eV. Comparative analysis shows that the oxygen adsorption energy on M4/Cr2O3–Ov dictates CO2 hydrogenation selectivity. Moreover, a smaller work function of metal strengthens O* binding and thereby promotes CH4 selectivity. Conversely, a bigger work function weakens O* adsorption and favors CO formation. These findings establish the work function of metals as an intrinsic, experimentally accessible descriptor for tuning CO2 hydrogenation selectivity, guiding the rational design of supported metal catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


