由没食子酸、尿素和氯化锌三元共晶溶剂合成的多孔碳的热化学和结构表征:对CO2吸附性能的见解
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
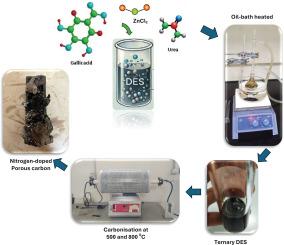
采用没食子酸、尿素和氯化锌(Ga:U:ZnCl 2)组成的三元深共晶溶剂(DES)合成了氮掺杂多孔碳(NPC)。在两种炭化温度(500°C和800°C)下进行合成,前驱体的摩尔比不同。FTIR分析证实了含氮官能团的掺入,正如NH和OH拉伸振动所表明的那样。TGA结果表明,尿素含量越低,NPC的热稳定性越好,其中NPC800-161的热回弹性最高。结构表征表明,随着炭化温度和尿素含量的升高,石墨化程度降低。XPS分析进一步证实了这些发现,表明在800°C时氮的种类分布发生了变化,吡啶- n占主导地位,增强了材料的吸附潜力。SEM和BET分析表明,碳化温度和尿素含量对其形貌和孔隙度有显著影响。NPC800-161具有蜂窝状多孔结构,BET比表面积为306.99 m²/g, CO₂吸附量为1.86316 mmol/g。这些结果强调了优化前驱体比例和碳化条件对于提高NPC在CO₂捕获、催化和能量储存方面的性能的重要性。NPC800-161作为一种有前景的环境和工业应用材料,展示了des衍生的NPCs在应对可持续发展挑战方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermochemical and structural characterization of porous carbon synthesized from a ternary deep eutectic solvent of gallic acid, urea, and zinc chloride: Insights into CO2 adsorption performance
A novel approach was developed for synthesizing nitrogen-doped porous carbon (NPC) using a ternary deep eutectic solvent (DES) composed of gallic acid, urea, and zinc chloride (Ga:U:ZnCl₂). The synthesis was performed at two carbonization temperatures (500 °C and 800 °C), with varying molar ratios of precursors. FTIR analysis confirmed the incorporation of nitrogen-containing functional groups, as indicated by N![]() H and O
H and O![]() H stretching vibrations. TGA results demonstrated that NPC thermal stability improved with lower urea content, with NPC800–161 exhibiting the highest thermal resilience. Structural characterization revealed that graphitization decreased with increasing carbonization temperature and urea content, as shown by XRD and Raman spectroscopy. XPS analysis further validated these findings, showing a shift in nitrogen species distribution, with pyridinic-N dominating at 800 °C, enhancing the material’s adsorption potential. SEM and BET analyses indicated that carbonization temperature and urea content significantly influenced morphology and porosity. NPC800–161, with a honeycomb-like porous structure, achieved a BET surface area of 306.99 m²/g and a CO₂ adsorption capacity of 1.86316 mmol/g. These results emphasize the importance of optimizing precursor ratios and carbonization conditions to enhance NPC performance for CO₂ capture, catalysis, and energy storage. NPC800–161 emerges as a promising material for environmental and industrial applications, demonstrating the potential of DES-derived NPCs in addressing sustainability challenges.
H stretching vibrations. TGA results demonstrated that NPC thermal stability improved with lower urea content, with NPC800–161 exhibiting the highest thermal resilience. Structural characterization revealed that graphitization decreased with increasing carbonization temperature and urea content, as shown by XRD and Raman spectroscopy. XPS analysis further validated these findings, showing a shift in nitrogen species distribution, with pyridinic-N dominating at 800 °C, enhancing the material’s adsorption potential. SEM and BET analyses indicated that carbonization temperature and urea content significantly influenced morphology and porosity. NPC800–161, with a honeycomb-like porous structure, achieved a BET surface area of 306.99 m²/g and a CO₂ adsorption capacity of 1.86316 mmol/g. These results emphasize the importance of optimizing precursor ratios and carbonization conditions to enhance NPC performance for CO₂ capture, catalysis, and energy storage. NPC800–161 emerges as a promising material for environmental and industrial applications, demonstrating the potential of DES-derived NPCs in addressing sustainability challenges.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


