Barapukuria烟煤在多种升温速率下的热分解动力学和热力学分析:对有效反应器设计的影响
IF 5.4
3区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
孟加拉国是一个人口稠密的国家,面临着重大的能源挑战,煤炭在加强能源安全方面发挥着至关重要的作用。为了优化煤炭利用,热重分析(TGA)等先进技术是必不可少的。本研究通过热重分析仪分析了煤样在N2环境下,在加热速率为10、20和30°C/min的条件下,揭示了多个热降解区域:水分蒸发、脱挥发和焦炭形成。动力学分析以脱挥发为中心,利用Coats-Redfern模型(CRM)评估了4种固态反应模型中的21种反应机理。脱挥发阶段在以下温度范围内发生:390-550°C, 415-605°C和440-680°C,分别在10,20和30°C/min下进行。三维输运扩散方程(DM3)的回归系数(R2)最大。频率因子(A)和活化能(Ea)分别为287.13 min−1和177.725kJ/mol。对脱挥发的热力学性质也进行了评价,Gibbs自由能(ΔG)在259.3 ~ 274.5 kJ mol-1之间变化,焓(ΔH)在163.7 ~ 179.25 kJ mol-1之间变化,熵(ΔS)在0.2103 ~ 0.2094 kJ mol- 1k -1之间变化。ICP-OES分析发现煤中存在显著的过渡态重金属(Fe、Pb、Zn、Ni、Cr、Cd),但未发现碱金属和碱土金属(K、Na、Ca、Mg)。如果没有aaem,就需要外部添加来提高炭的反应性,降低着火温度,降低活化能。本研究结果为设计高效反应堆、优化煤炭利用和推进能源可持续性战略提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
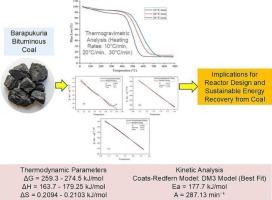
Thermal decomposition kinetics and thermodynamic analysis of Barapukuria bituminous coal at multi-heating rates using model-fitting approach: implications for effective reactor design
Bangladesh, a densely populated country, faces significant energy challenges, with coal playing a crucial role in enhancing energy security. To optimize coal utilization, advanced techniques like thermogravimetric analysis (TGA) are essential. This study analyzed coal samples via TGA in a N2 environment at heating rates (HRs) of 10, 20, and 30 °C/min, revealing multi thermal degradation regions: moisture evaporation, devolatilization, and char formation. Kinetic analysis centered on devolatilization, utilizing the Coats-Redfern model (CRM) to assess 21 reaction mechanisms within four models of solid-state reactions. The devolatilization phase takes place across the following temperature ranges: 390–550 °C, 415–605 °C, and 440–680 °C at HRs of 10, 20, and 30 °C/min, respectively. The three-dimensional transport diffusion equation (DM3) exhibited the greatest regression coefficient (R2). The frequency factor (A) and activation energy (Ea) were determined as 287.13 min−1 and 177.725kJ/mol, respectively. Thermodynamic properties of devolatilization were also assessed, with Gibbs free energy (ΔG) varying between 259.3 to 274.5 kJ mol−1, enthalpy (ΔH) from 163.7 to 179.25 kJ mol−1, and entropy (ΔS) from 0.2103 to 0.2094 kJ mol-1K−1. ICP-OES analysis detected significant transition/heavy metals (Fe, Pb, Zn, Ni, Cr, Cd) but no alkali and alkaline earth metals (AAEMs) (K, Na, Ca, Mg) in the coal. The absence of AAEMs necessitates external addition to improving char reactivity, lower ignition temperatures, and reduce activation energy. The outcome of this research offers valuable insights for designing efficient reactors, optimizing coal utilization, and advancing energy sustainability strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Thermal Science and Engineering Progress
Chemical Engineering-Fluid Flow and Transfer Processes
CiteScore
7.20
自引率
10.40%
发文量
327
审稿时长
41 days
期刊介绍:
Thermal Science and Engineering Progress (TSEP) publishes original, high-quality research articles that span activities ranging from fundamental scientific research and discussion of the more controversial thermodynamic theories, to developments in thermal engineering that are in many instances examples of the way scientists and engineers are addressing the challenges facing a growing population – smart cities and global warming – maximising thermodynamic efficiencies and minimising all heat losses. It is intended that these will be of current relevance and interest to industry, academia and other practitioners. It is evident that many specialised journals in thermal and, to some extent, in fluid disciplines tend to focus on topics that can be classified as fundamental in nature, or are ‘applied’ and near-market. Thermal Science and Engineering Progress will bridge the gap between these two areas, allowing authors to make an easy choice, should they or a journal editor feel that their papers are ‘out of scope’ when considering other journals. The range of topics covered by Thermal Science and Engineering Progress addresses the rapid rate of development being made in thermal transfer processes as they affect traditional fields, and important growth in the topical research areas of aerospace, thermal biological and medical systems, electronics and nano-technologies, renewable energy systems, food production (including agriculture), and the need to minimise man-made thermal impacts on climate change. Review articles on appropriate topics for TSEP are encouraged, although until TSEP is fully established, these will be limited in number. Before submitting such articles, please contact one of the Editors, or a member of the Editorial Advisory Board with an outline of your proposal and your expertise in the area of your review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


