高性能半固态锂金属电池中聚(1,3-二恶烷)交联和氟化的原位协同设计
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
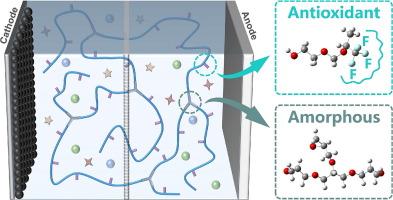
原位聚(1,3-二氧氧烷)(PDOL)基电解液因其对锂负极的高稳定性和工艺简单而受到锂金属电池研究的广泛关注。然而,它仍然面临着固有离子电导率低、电化学窗口窄、热稳定性差等缺陷。针对上述问题,提出了一种交联-氟化PDOL分子设计策略。非晶交联结构通过抑制长链结晶有效提高离子电导率。特别是抗氧化剂-CF3基团,稳定的交联结构和减少的末端羟基显著提高了电化学氧化稳定性,具有4.7 V的极好的高压窗口。此外,所设计的电解质还表现出明显改善的热稳定性,在120℃下保持5 min不变形。此外,半固态NCM811||锂电池在0.5 c下循环150次后的容量保持率为88.8%,即使与工作电压为4.5 V的NCM622正极组合,在0.5 c下循环100次后的容量保持率仍可达到85.3%。活性物质负载量为9 mg/cm2的0.1 Ah NCM811||锂袋电池也表现出良好的循环稳定性和工作能力,具有良好的实际应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In-situ collaborative design of crosslinking and fluorination toward poly(1,3-dioxolane) for high-performance semi-solid lithium metal batteries
In-situ poly(1,3-dioxolane) (PDOL)-based electrolyte has received extensive attention in the research of lithium metal batteries due to its high stability to lithium anode and simple processing. However, it is still faced with defects such as low intrinsic ionic conductivity, a narrow electrochemical window, and poor thermal stability. A crosslinking and fluorination molecular design strategy toward PDOL is proposed to tackle the issues above. The amorphous crosslinked structure effectively improves ionic conductivity by inhibiting long-chain crystallization. Especially, the antioxidant –CF3 groups, stable crosslinked structure, and reduced terminal hydroxyl groups significantly enhance the electrochemical oxidation stability with a superb high-voltage window of 4.7 V. In addition, the designed electrolyte also exhibits obviously improved thermal stability with no deformation at 120 °C for 5 min. Furthermore, the semi-solid NCM811||Li batteries exhibit a favourable capacity retention of 88.8 % after 150 cycles at 0.5 C. Even assembled with NCM622 cathode working at 4.5 V, the semi-solid batteries can still show a satisfactory capacity retention of 85.3 % after 100 cycles at 0.5 C. Also, a 0.1 Ah NCM811||Li pouch cell with active materials loading of 9 mg/cm2 demonstrates satisfactory cycling stability and working ability, which shows promising practical application prospects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


