环糊精结构和电荷对优化核酸传递的影响:单体、二聚体和聚合物的比较
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
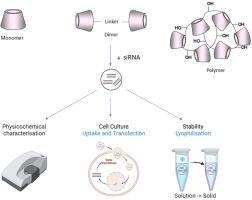
环糊精(CDs)是一种环状低聚糖,由于其独特的环形结构和可修饰的主羟基和仲羟基,为药物输送应用提供了高度可调的平台。由于这些独特的特征,CDs已成为递送核酸治疗药物的有希望的非病毒载体,包括反义寡核苷酸(ASOs)和小干扰RNA (siRNA)。在这项研究中,我们评估和比较了用于siRNA递送的β-环糊精(β-CD)基单体、二聚体和阳离子聚合物的物理化学和生物学性能。该结构包括一个通过三唑基团连接的头对头的β-CD二聚体,以及两个以季铵(qa -聚合物)或伯胺(pa -聚合物)基团功能化的β-CD基聚合物,其阳离子官能团的取代度分别为3.5和7。虽然单体和二聚体在测试条件下没有表现出siRNA的络合作用,但琼脂糖凝胶证实,QA-和pa -聚合物都能有效地络合siRNA,在聚合物:siRNA质量比(MR)为5:1、7.5:1和10:1时,在150-200 nm范围内形成稳定的纳米颗粒(NPs)。动态光散射(DLS)显示,聚合物的粒径分布较窄(150 ~ 200 nm, qa -聚合物- np的PDI为0.2,pa -聚合物- np的PDI为0.4),zeta电位在26 ~ 37 mV范围内,具有良好的胶体稳定性。在4°C下长期储存并冷冻干燥(不使用冷冻保护剂),然后再重悬,与qa聚合物相比,pa聚合物NPs具有更高的稳定性。体外评估,使用a549荧光素酶(A549luc)肺癌细胞显示,两种聚合物载体在工作浓度(20 pmol siRNA/孔)下无毒,细胞滴度-荧光™细胞活力测定显示,细胞活力保持≥80%。流式细胞仪的细胞摄取研究表明,qa聚合物的内化程度最低,而pa聚合物的内化程度高达40%,通过ONE-Glo™EX荧光素酶测定,导致大约40%的基因敲除。这些发现强调了聚合物结构和表面电荷化学对细胞相互作用和基因沉默效率的影响,pa -聚合物的增强性能可能归因于其可质子化胺及其促进内体逃逸的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The impact of cyclodextrin architecture and charge for optimized nucleic acid delivery: A comparison of monomers, dimers, and polymers
Cyclodextrins (CDs) are cyclic oligosaccharides that offer a highly tuneable platform for drug delivery applications, owing to their unique toroidal architecture and modifiable primary and secondary hydroxyl groups. Due to these distinctive features, CDs have emerged as promising non-viral vectors for the delivery of nucleic acid therapeutics, including antisense oligonucleotides (ASOs) and small interfering RNA (siRNA). In this study, we evaluate and compare the physicochemical and biological performance of β-cyclodextrin (β-CD)-based monomer, dimers and cationic polymers engineered for siRNA delivery. The constructs include a head-to-head β-CD dimer linked via a triazole moiety, as well as two β-CD-based polymers functionalized with either quaternary ammonium (QA-polymer) or primary amine (PA-polymer) groups, with degrees of substitution of 3.5 and 7, respectively, for the cationic functionalities. Although the monomer and dimer did not show siRNA complexation under the tested conditions, both QA- and PA-polymers efficiently complexed siRNA, as confirmed by agarose gel, forming stable nanoparticles (NPs) in the 150–200 nm size range at polymer:siRNA mass ratios (MR) of 5:1, 7.5:1, and 10:1. Dynamic light scattering (DLS) revealed narrow size distributions (150 – 200 nm, PDI 0.2 for QA-polymer-NP and 0.4 for PA-polymer-NP) and zeta potentials in the range of 26–37 mV, indicating favourable colloidal stability. Long-term storage at 4 °C and freeze-drying (without the use of cryoprotectants) followed by resuspension demonstrated superior stability of PA-polymer NPs compared to their QA-polymer counterparts. In vitro evaluation, using A549-luciferase (A549luc) lung carcinoma cells showed that both polymeric carriers were non-toxic at working concentrations (20 pmol siRNA/well), maintaining ≥80 % cell viability as demonstrated by CellTiter-Fluor™ Cell Viability Assay. Cellular uptake studies with flow cytometry indicated minimal internalization for the QA-polymer, whereas the PA-polymer achieved up to 40 % uptake, resulting in approximately 40 % gene knockdown, as assessed by ONE-Glo™ EX Luciferase Assay. These findings underscore the influence of polymer architecture and surface charge chemistry on cellular interaction and gene silencing efficiency, with the enhanced performance of the PA-polymer potentially attributable to its protonatable amines and their capacity to promote endosomal escape.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


