非那雄胺在β-CD和三甲基β-CD配合物中的分子相互作用和释放机制:计算和实验方法
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-10-04
DOI:10.1016/j.carpta.2025.101025
引用次数: 0
摘要
非那雄胺(Fin)的低水溶性对其在患者中的有效使用提出了重大挑战。为了解决这一问题,制备了三甲基β-环糊精(TM-β-CD)包封Fin,改善其水溶性和释放性,并改善其其他物理特性。此外,制备了β-CD与Fin的包合物,以提供两种载体之间的比较。采用BET、XRD、FT-IR、TGA、FE-SEM和DLS等技术对配合物进行了表征。采用GaussView 5.0.8软件对包封药物的形成过程进行了计算研究。结果表明,β-CD/Fin包封率较高。此外,两种载体对Fin的体外释放研究表明,β-CD/Fin具有可持续的累积释放。动力学研究揭示了TM-β-CD/Fin和β-CD/Fin的非菲克斯释放机制和case II释放机制。这些结果表明,TM-β-CD可用于改善Fin水溶性的应用,而β-CD可用于延长释放过程。从计算方法中获得的数据证实了实验结果,并提供了对这些现象背后的基本原理的见解。此外,模拟研究表明,Fin通过其吡啶环进入CDs的空腔,并与TM-β-CD形成氢键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
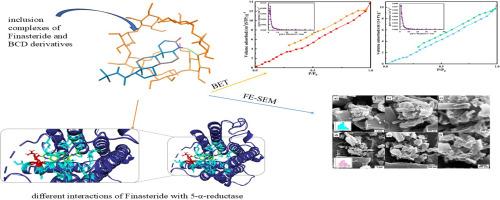
Molecular interactions and release mechanisms of Finasteride in β-CD and Trimethyl-β-CD complexes: A computational and experimental approach
The low water solubility of Finasteride (Fin) presents a significant challenge to its effective use in patients. To address this issue, the trimethyl-β-cyclodextrin (TM-β-CD) was prepared to encapsulate Fin, improving its aqueous solubility and release, along with other physical characteristics. Additionally, an inclusion complex of β-CD with Fin was prepared to provide a comparison between both carriers. The complexes were characterized using BET, XRD, FT-IR, TGA, FE-SEM, and DLS techniques. The formation of the encapsulated drug was studied computationally through GaussView 5.0.8. The encapsulation efficiency study showed a higher yield for β-CD/Fin. Furthermore, the in vitro release study of Fin from both carriers demonstrated a sustainable cumulative release for β-CD/Fin. The kinetic study revealed a non-Fickian and case II release mechanism for TM-β-CD/Fin and β-CD/Fin. These findings suggest TM-β-CD for applications requiring improved Fin water solubility and β-CD to prolong the release process for different purposes. The data obtained from computational methods confirmed the experimental findings and provided insight into the rationale behind these phenomena. Furthermore, a simulation study suggested that Fin enters the CDs` cavities via its pyridine ring and forms hydrogen bonds with TM-β-CD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


