天然长链烷基间苯二酚的直接和绿色合成
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
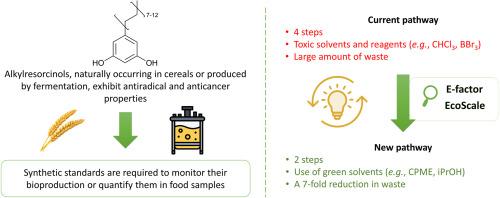
烷基间苯二酚是一种天然酚类化合物,具有有趣的特性(如抗氧化、抗癌)。为了监测它们的生物生产或在食品样品中量化它们,需要制定标准。然而,由于其奇数长烷基链(C > 15),只有少数几种合成路线可用,最有效的方法依赖于Wittig反应。为了提供更可持续的合成路线,进行了两项主要修改:(i)将起始原料从3,5-二甲氧基苯甲醛改为3,5-二苯氧基苯甲醛,从而无需使用高毒性试剂(如BBr3或HBr)进行去甲基化;(ii)溶剂系统被更环保、更安全的替代品——环戊基甲基醚和异丙醇——取代了以前出版物中使用的甲苯、二甲基亚砜和二氯甲烷。该策略还可以用两个一锅两步反应取代四步反应序列,使C:19和C:21烷基间苯二酚的总收率分别达到66%和60%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Straightforward and greener synthesis of naturally occurring long chain alkylresorcinols
Alkylresorcinols are natural phenolipids exhibiting interesting properties (e.g., antioxidant, anticancer). To monitor their bioproduction or quantify them in food samples, standards are required. However, due to their odd long alkyl chain (C > 15), only a few synthetic routes are available, with the most efficient ones relying on the Wittig reaction. To provide a more sustainable synthetic route, two major modifications were implemented: (i) the starting material was changed from 3,5-dimethoxybenzaldehyde to 3,5-dibenzyloxybenzaldehyde, thereby eliminating the need for demethylation with highly toxic reagents such as BBr3 or HBr; and (ii) the solvent system was replaced with greener and safer alternatives - cyclopentyl methyl ether and isopropyl alcohol - instead of the toluene, dimethyl sulfoxide, and dichloromethane used in previous publications. This strategy also enabled the replacement of a four-step sequence with two one-pot two-step reactions, resulting in good overall yields of 66 % and 60 % for C:19 and C:21 alkylresorcinols, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


