无紫外源深度降解碳氢化合物:TiO2和ZnO纳米催化剂的效率
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
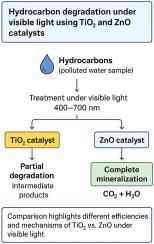
该研究探索了两种常见的金属氧化物,二氧化钛(TiO2)和氧化锌(ZnO),在用于清洁富含石油碳氢化合物的钻井泥浆时的表现。实验在实验室矿物油和凝析油的混合物上进行,在可见光下,使用不同的催化剂负载(1、5和10 g L−1)。这两种材料的结果截然不同。二氧化钛只导致碳氢化合物的部分分解,留下一些含氧的碎片,如醇、酮和酸。另一方面,ZnO表现出更强的响应:在5 g L−1时,碳氢化合物含量几乎完全下降(去除率≈99%),并且碳平衡证实大部分物质已经转化为CO2和水。额外的测试有助于解释这种差异。EPR和XPS分析支持的自由基捕获和氧净化实验表明,ZnO含有更多能够激活氧的结构缺陷,从而导致活性自由基的产生。显微镜也显示处理后两种材料的表面有明显的变化。从实用的角度来看,该方法简单,不涉及有机溶剂;通过离心回收纳米颗粒,总体能量需求适中(每毫克碳氢化合物去除约6.3 Wh)。这些结果表明,ZnO可能是一种可靠且可持续的选择,仅使用可见光处理含油废物,而不需要紫外线灯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Advanced degradation of hydrocarbons without UV source: Efficiency of TiO2 and ZnO nanocatalysts
The study explores how two common metal oxides, titanium dioxide (TiO2) and zinc oxide (ZnO), behave when used to clean drilling muds rich in petroleum hydrocarbons. Tests were carried out on a laboratory mixture of mineral oil and condensate, under visible light, using different catalyst loads (1, 5, and 10 g L−1).
The outcomes were quite distinct for the two materials. TiO2 only led to partial breakdown of the hydrocarbons, leaving behind several oxygenated fragments such as alcohols, ketones and acids. ZnO, on the other hand, showed a much stronger response: at 5 g L−1 the hydrocarbon content dropped almost completely (≈99 % removal), and the carbon balance confirmed that most of the material had been converted into CO2 and water.
Additional tests helped explain this difference. Radical trapping and oxygen purging experiments, supported by EPR and XPS analyses, indicated that ZnO contains more structural defects able to activate oxygen, which in turn leads to a higher production of reactive radicals. Microscopy also revealed clear surface changes on both materials after treatment.
From a practical point of view, the method is simple and does not involve organic solvents; the nanoparticles were recovered by centrifugation, and the overall energy requirement was modest (∼6.3 Wh per milligram of hydrocarbons removed). These results suggest that ZnO could be a reliable and sustainable option for treating oily wastes using only visible light, without the need for UV lamps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


