4′,5-二甲氧基-3-羟基黄酮分子内激发态质子转移反应:5-OCH3基团对质子分子间氢键的部分屏蔽及其对ESIPT速率和荧光光谱的影响
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-10-03
DOI:10.1016/j.jphotochem.2025.116817
引用次数: 0
摘要
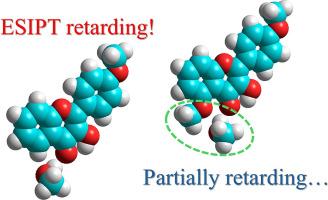
我们继续研究已知最快的光化学反应——激发态分子内质子转移(ESIPT)的分子内和分子间调节因子,以3-羟基黄酮(3HF)系列为例,在3HF核心的第5位引入甲氧基,目的是检查其对4位羰基与质子分子间氢键的潜在屏蔽作用。这种分子间氢键被认为是3-羟基黄酮ESIPT反应的主要阻碍因素。对4 ',5-二甲氧基-3-羟基黄酮(45DM3HF)的取代效应进行了理论和实验研究:荧光光谱和寿命使我们能够估计在一系列质子溶剂中的ESIPT速率,并将其与5位无取代基的模型化合物的数据进行比较。理论和实验都揭示了分子间氢键的部分抑制作用,因此标题化合物中的ESIPT反应对质子供体和/或非质子环境中的湿度的敏感性较低。5-甲氧基的额外作用是没有典型的到大多数3hf基态阴离子的形成,这在45DM3HF的吸收光谱和荧光光谱中都没有记录。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Excited state intramolecular proton transfer reaction in 4′,5-dimethoxy-3-hydroxyflavone: partial shielding of intermolecular H-bonding with protic molecules by 5-OCH3 group and its impact on ESIPT rate and fluorescence spectra
Continuing our investigations of intra- and intermolecular factors regulating the fastest known photochemical reaction, the excited state intramolecular proton transfer (ESIPT), on the example of 3-hydroxyflavone (3HF) series, methoxy group was introduced in position 5 of 3HF core with the aim to check its potential shielding effect for carbonyl group in position 4 from intermolecular H-bonding with protic molecules. Such an intermolecular hydrogen bond was considered as the main retarding factor for the ESIPT reaction of 3-hydroxyflavones. The effect of such substitution was studied both theoretically and experimentally for 4′,5-dimethoxy-3-hydroxyflavone (45DM3HF): fluorescence spectra and lifetimes allow us to estimate ESIPT rates in a series of protic solvents and compare them with the data of model compound without substituents in position 5. Partial inhibition of intermolecular H-bonding was revealed both theoretically and experimentally, owing to which ESIPT reaction in the title compound demonstrates less pronounced sensitivity to proton donors and/or humidity in aprotic surroundings. The additional effect of 5-methoxy group is the absence of typical to most 3HFs ground state anionic species formation, which were not registered both in the absorption and fluorescence spectra of 45DM3HF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


