超声促进新型1,3,4 -恶二唑绿色合成:抗食管癌评价、细胞凋亡形态学、dft计算、硅ADME和分子对接研究
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
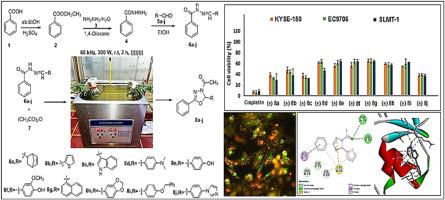
采用超声技术合成了一系列新的3-乙酰- 2,3 -二氢- 1,3,4 -恶二唑衍生物(8a-j),探讨其对食管癌的抗增殖作用。通过1H和13C NMR、FTIR和质谱等不同的光谱工具对目标衍生物的化学结构进行了验证。超声波技术操作简单,无溶剂,可扩展收率高,节能,符合绿色化学的要求。用对映选择性高效液相色谱技术分离了目标衍生物中的潜在对映体。HPLC分析证实这些衍生物为外消旋混合物。目标衍生物在三种食道细胞系(KYSE-150、EC9706和SLMT-1)上进行了实验。有趣的是,分离的(R)-(+)对映体比外消旋体和相应的(S)-(−)对映体表现出更高的抗增殖活性。(R)-(+)对映体中,(R)-(+) 8a、(R)-(+) 8b、(R)-(+) 8c和(R)-(+) 8j的IC50值范围为1.68±0.01 ~ 2.89±0.11 μM,比顺铂的IC50值(1.32±0.01 ~ 2.63±0.21 μM)高。通过双染色法(膜联蛋白V-FITC和PI),形态学数据表明,与它们在NE-3和HET-1A(正常细胞)中的作用相比,最有效的(R)-(+)对映体能够以一种特有的途径触发上述细胞的凋亡过程。在DFT计算中,发现目标衍生物(8a-j)中的主要(R)-(+)对映体比对应的(S)-(−)对映体更稳定。对最有效的衍生物(8a, 8b, 8c和8j)的ADME预测没有显示Lipinski的违规,因为它们具有良好的口服药物相似标准。上述衍生物与一些靶蛋白(PDB ID: 2LEO、5HZN和6DUK)的对接得分证实了与上述蛋白的良好结合作用。总的来说,最有效的衍生物作为食管癌疾病的有希望的候选者出现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrasonic-promoted green synthesis of new 1, 3, 4-oxadiazoles: Anti-esophageal cancer evaluation, apoptosis morphology, DFT-calculations, in silico ADME, and molecular docking study
A new series of 3-acetyl-2, 3-dihydro-1, 3, 4-oxadiazole derivatives (8a-j) was synthesized under ultrasonic technique to explore their antiproliferative efficacy towards esophageal cancer. The chemical structures of the target derivatives were authenticated by different spectroscopic tools including 1H and 13C NMR, FTIR, and Mass spectra. Using ultrasonic technique offers a simple operation, high scalable yields solvent-free, saving energy, and being agreement with green chemistry. The potential enantiomers found in the target derivatives were separated by an enantioselective HPLC technique. The HPLC analysis confirmed these derivatives are a racemic mixture. The target derivatives were tested against three esophageal cell lines (KYSE-150, EC9706, and SLMT-1). Interestingly, the separated (R)-(+) enantiomers are found to show high antiproliferative activity than their racemic and corresponding (S)-(−) forms. Among the (R)-(+) enantiomers, (R)-(+) 8a, (R)-(+) 8b, (R)-(+) 8c and (R)-(+) 8j showed high potency with a range of IC50 values (1.68 ± 0.01 to 2.89 ± 0.11 μM) compared to cisplatin (IC50 = 1.32 ± 0.01 to 2.63 ± 0.21 μM). By using a dual staining assay (annexin V-FITC and PI), the morphological data demonstrated that the most potent (R)-(+) enantiomers are able to trigger the apoptotic process of the aforementioned cells in a characteristic pathway compared with their effects in the NE-3 and HET-1A (normal cells). In DFT calculations, the findings revealed that the major (R)-(+) enantiomers found in the target derivatives (8a-j) were more stable compared to their corresponding (S)-(−) enantiomers. The ADME predictions of the most potent derivatives (8a, 8b, 8c, and 8j) displayed no Lipinski's violation as they have favorable oral drug-likeness criteria. The docking scores of the above derivatives with some targeting proteins (PDB ID: 2LEO, 5HZN, and 6DUK), confirmed favorable binding interactions with the aforementioned proteins. Overall, the most potent derivatives emerge as a promising candidate for esophageal cancer diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


