回收废锂离子电池正极材料合成高效CO2秸秆气化镍钴锰催化剂
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
在碳中和目标的推动下,二氧化碳气化技术的应用已经显示出显著的环境和经济效益。本研究利用废旧锂离子电池正极材料LiNi0.5Co0.2Mn0.3、LiCoO2、LiMn2O4合成了高效的二氧化碳辅助秸秆气化镍钴锰催化剂。在实验室规模的固定床气化反应器上进行了气化实验,并利用XRD、XPS、SEM、TGA等表征方法研究了催化剂结构和理化性质的演变。结果表明,以LiNi0.5Co0.2Mn0.3为原料制备的催化剂,合成气产率达到747.80 mL/g,比未添加催化剂的对照组提高了134%;使用LiCoO2将钴含量调整为40%后,合成气收率进一步提高至865.24 mL/g,而焦油产量显著下降84%至4.4 wt%, 10个循环后活性仅下降23.3%。其优异的性能源于Ni、Co、Mn的协同作用:镍促进二氧化碳分解,钴增强电子传导,锰稳定骨架结构。优化钴含量有利于C-C键解理和Boudouard反应,从而促进焦油裂解和一氧化碳生成,并通过Ni-Co-Mn协同重整有效抑制甲烷生成。本研究建立了“废电池-催化剂-生物质气化”闭环策略,实现了废锂电池的回收利用和合成气的高效生产,为废锂电池高价值利用和催化生物质气化的工业应用提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
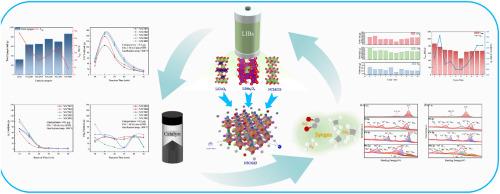
Recycling of waste lithium-ion battery cathode materials to synthesize nickel-cobalt-manganese catalysts for efficient CO2 straw gasification
Driven by carbon neutrality goals, the application of carbon dioxide gasification technology has demonstrated significant environmental and economic benefits. This study utilized waste lithium-ion battery cathode materials (LiNi0.5Co0.2Mn0.3, LiCoO2, LiMn2O4) to synthesize highly efficient nickel-cobalt-manganese catalysts for carbon dioxide-assisted straw gasification. Gasification experiments were conducted using a laboratory-scale fixed-bed gasification reactor, and the evolution of the catalyst's structure and physicochemical properties were investigated using characterization methods such as XRD, XPS, SEM, and TGA. The results showed that the catalyst prepared using LiNi0.5Co0.2Mn0.3 as raw material increased the synthesis gas yield to 747.80 mL/g, representing a 134 % increase compared to the control group without catalyst addition; After adjusting the cobalt content to 40 % using LiCoO2, the synthesis gas yield further increased to 865.24 mL/g, while tar production decreased significantly by 84 % to 4.4 wt%, and activity only declined by 23.3 % after ten cycles. Its outstanding performance stems from the synergistic interaction of Ni, Co, and Mn: nickel promotes carbon dioxide decomposition, cobalt enhances electron conduction, and manganese stabilizes the framework structure. Optimizing cobalt content facilitates C–C bond cleavage and the Boudouard reaction, thereby enhancing tar cracking and carbon monoxide production, and effectively inhibits methane formation through Ni-Co-Mn synergistic reforming. This study establishes a closed-loop “waste battery-catalyst-biomass gasification” strategy, which enables the recycling of waste lithium batteries and the efficient production of synthesis gas, providing a reference for industrial application of high-value utilization of waste lithium batteries and catalytic biomass gasification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


