间充质干细胞衍生培养条件培养基在急性移植物抗宿主病中的剂量-反应免疫调节作用
IF 3.5
3区 环境科学与生态学
Q3 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
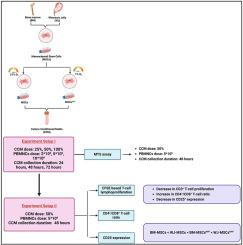
基于间充质干细胞的治疗面临着挑战,人们对间充质干细胞衍生的培养条件培养基(CCM)作为一种无细胞替代疗法产生了兴趣。我们的研究旨在优化CCM的剂量和采集时间,以提高其对aGVHD的治疗效果,同时规范CCM与CD3+ t细胞相互作用的共培养条件。材料和方法从BM和WJ中分离出人间充质干细胞,随后在三气培养箱中缺氧条件(1% O2)预处理24小时。培养条件培养基(CCM)分别于24、48和72 h从未处理(MSCs)和缺氧预处理的MSCs (MSCsHYP)中收集,并使用0.2 μm膜过滤器过滤。从aGVHD患者的pbmnc中分离CD3+ t细胞。这些t细胞以不同密度(2∗106,5∗106和10∗106细胞/ml)与不同浓度的CCM(25%, 50%和100%)共培养,并使用MTS法评估细胞增殖。此外,在CD3+ t细胞和CCM的二维共培养模型中,使用流式细胞术评估CD3+ t细胞的增殖和激活状态。我们的研究结果显示,无论MSCs来源如何,在48小时收集的CCM以50%的浓度对CD3+ t细胞增殖具有最明显的抑制作用,特别是当密度为5∗106个细胞/ml时。低氧预处理显著增强免疫调节作用,WJ-MSCsHYP-CCM在抑制t细胞增殖、提高CD4+/CD8+ t细胞比例、降低CD4+ t细胞活化方面优于BM-MSCsHYP-CCM。结论优化CCM的采集时机和浓度对发挥治疗潜力至关重要。我们的研究为开发标准化、可扩展和有效的aGVHD无细胞疗法铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dose-response immunomodulatory effects of Mesenchymal stem cell-derived culture-conditioned media in acute Graft-versus-Host Disease
Background
Mesenchymal stem cell-based therapy faces challenges that have driven interest in MSCs-derived culture-conditioned media (CCM) as a cell-free alternative. Our study aims to optimize the dose and collection timing of CCM to enhance its therapeutic efficacy in aGVHD, while also standardizing co-culture conditions for CD3+ T-cell interaction with CCM.
Material and methods
Human MSCs were isolated from BM and WJ and subsequently preconditioned under hypoxic conditions (1 % O2) for 24 h in a tri-gas incubator. Culture-conditioned media (CCM) were collected from both naive (MSCs) and hypoxia-preconditioned MSCs (MSCsHYP) at 24, 48, and 72 h and filtered using a 0.2 μm membrane filter. CD3+ T-cell were isolated from PBMNCs derived from aGVHD patients. These T-cell were co-cultured at varying densities (2∗106, 5∗106, and 10∗106 cells/ml) with different concentrations of CCM (25 %, 50 %, and 100 %), and cell proliferation was assessed using the MTS assay. Furthermore, CD3+ T-cell proliferation and activation status were evaluated in a 2D co-culture model of CD3+ T-cell and CCM using flow cytometry.
Results
Our findings revealed that CCM collected at 48 h, at a 50 % concentration, exerted the most pronounced inhibitory effect on CD3+ T-cell proliferation, particularly at a density of 5∗106 cells/ml, irrespective of the MSCs source. Hypoxia preconditioning significantly enhanced the immunomodulatory effects, with WJ-MSCsHYP-CCM demonstrating superior efficacy in suppressing T-cell proliferation, increasing the CD4+/CD8+ T-cell ratio, and reducing CD4+ T-cell activation compared to BM-MSCsHYP-CCM.
Conclusion
These results emphasize the critical role of optimizing CCM collection timing and concentration to maximize therapeutic potential. Our study paves the way for the development of standardized, scalable, and effective cell-free therapies for aGVHD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Regenerative Therapy
Engineering-Biomedical Engineering
CiteScore
6.00
自引率
2.30%
发文量
106
审稿时长
49 days
期刊介绍:
Regenerative Therapy is the official peer-reviewed online journal of the Japanese Society for Regenerative Medicine.
Regenerative Therapy is a multidisciplinary journal that publishes original articles and reviews of basic research, clinical translation, industrial development, and regulatory issues focusing on stem cell biology, tissue engineering, and regenerative medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


