KHCO3和K2CO3抑制铝粉尘爆炸:抑制剂粒度和热化学协同作用的影响
IF 4.6
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
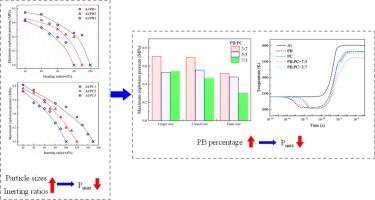
研究了碳酸钾盐(KHCO3和K2CO3)对铝粉尘爆炸的抑制作用。缓蚀剂的变量主要包括粒径、惰化比和混合策略。在20 L球形爆炸系统中进行的实验表明,最大爆炸压力(Pmax)随惰化比的增加或抑制剂粒径的减小而显著降低。添加KHCO3比添加K2CO3更有效地降低了Pmax,在70% wt%的情况下几乎消除了Pmax。与KHCO3相比,当KHCO3/K2CO3为7:3时,Pmax降低23%。热特性分析、爆炸残余分析和数值模拟分析表明,两种抑制剂降低铝爆炸压力的方法不同。KHCO3主要在铝爆炸的早期阶段起作用,而K2CO3则在化学上参与了氧自由基的竞争。阐明了两种缓蚀剂的协同作用。这些发现强调了优化粒径、惰化比和混合配方的关键作用,以最大限度地提高抑制效率和经济可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Aluminium dust explosion suppression by KHCO3 and K2CO3: The influence of the inhibitor's particle size and thermochemical synergy
This study investigated the effects of potassium carbonate salts (KHCO3 and K2CO3) on the suppression of aluminium dust explosions. The inhibitor variables mainly include the particle size, the inerting ratio, and the mixing strategy. Experiments in a 20 L spherical explosion system revealed that the maximum explosion pressure (Pmax) decreased significantly with increasing inerting ratios or with decreasing inhibitor particle sizes. Adding KHCO3 reduced Pmax more effectively than adding K2CO3, which achieved near extinction at 70 wt%. Compared with KHCO3, a 7:3 KHCO3/K2CO3 mixture at 60 wt% lowered Pmax by 23 %. Thermal characteristic analyses, explosion residue analyses, and numerical modelling analyses indicated that the two inhibitors had different methods for reducing the aluminium explosion pressure. KHCO3 primarily acted in the early stage of the aluminium explosion, whereas K2CO3 chemically participated in oxygen radical competition. The synergistic effects of the two inhibitor mixtures were clarified. These findings highlight the critical role of optimising the particle size, inerting ratios, and hybrid formulations to maximise the suppression efficiency and economic viability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Powder Technology
工程技术-工程:化工
CiteScore
9.90
自引率
15.40%
发文量
1047
审稿时长
46 days
期刊介绍:
Powder Technology is an International Journal on the Science and Technology of Wet and Dry Particulate Systems. Powder Technology publishes papers on all aspects of the formation of particles and their characterisation and on the study of systems containing particulate solids. No limitation is imposed on the size of the particles, which may range from nanometre scale, as in pigments or aerosols, to that of mined or quarried materials. The following list of topics is not intended to be comprehensive, but rather to indicate typical subjects which fall within the scope of the journal's interests:
Formation and synthesis of particles by precipitation and other methods.
Modification of particles by agglomeration, coating, comminution and attrition.
Characterisation of the size, shape, surface area, pore structure and strength of particles and agglomerates (including the origins and effects of inter particle forces).
Packing, failure, flow and permeability of assemblies of particles.
Particle-particle interactions and suspension rheology.
Handling and processing operations such as slurry flow, fluidization, pneumatic conveying.
Interactions between particles and their environment, including delivery of particulate products to the body.
Applications of particle technology in production of pharmaceuticals, chemicals, foods, pigments, structural, and functional materials and in environmental and energy related matters.
For materials-oriented contributions we are looking for articles revealing the effect of particle/powder characteristics (size, morphology and composition, in that order) on material performance or functionality and, ideally, comparison to any industrial standard.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


