低成本回流冷凝-合成磷酸钴镍作为HER和OER的超低过电位电极
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
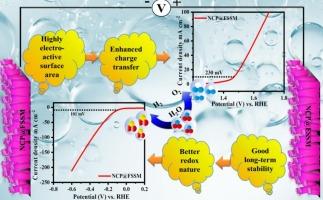
最近,人们越来越关注创造具有出色的水分解能力的经济实惠的纳米结构电极材料,作为贵金属基材料的有希望的替代品。因此,设计有效的双功能水分解电极对全球能源领域的研究人员来说是一个重大挑战。基于这一动机,在本研究中,我们采用回流冷凝沉积技术,在柔性不锈钢网(NCP@FSSM)上简单高效地合成了磷酸镍钴。合成的电极在10 mA cm−2下的析氧反应(OER)和析氢反应(HER)分别提供230 mV和101 mV的超低过电位,使其成为整体水分解的高性能候选材料。此外,长期耐用性测试表明,NCP@FSSM电极在OER和HER下的工作时间分别超过80小时和50小时,具有强大的运行稳定性,满足了商业可行性的关键要求。NCP@FSSM电极具有低过电位、高电化学表面积(ECSA)、高周转频率(TOF)数、良好的法拉第效率和较高的质量活性等优越的电化学特性,从而实现了高效的电催化。此外,独特的无粘结剂纳米棒结构确保了活性位点的密集分布以及镍、钴和磷之间的协同相互作用,从而优化了HER和OER性能。上述结果表明NCP@FSSM电极是一种很有前途的双功能水分解电极。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Low-cost reflux condensation-synthesized nickel cobalt phosphate as an ultra-low overpotential electrode for HER and OER
Recently, there has been a growing focus on creating affordable nanostructured electrode materials with outstanding water-splitting capabilities as a promising replacement for precious metal-based materials. Consequently, designing effective bifunctional electrodes for water splitting presents a significant challenge for researchers working in the energy sector around the globe. With this motivation, in this study, we report a facile and efficient synthesis of nickel cobalt phosphate on flexible stainless steel mesh (NCP@FSSM), employing a reflux condensation deposition technique. The as-synthesized electrode delivers ultra-low overpotentials of 230 mV and 101 mV at 10 mA cm−2 for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), respectively, positioning it as a high-performance candidate for overall water splitting. Moreover, long-term durability tests reveal robust operational stability with the NCP@FSSM electrode maintaining performance over 80 h for OER and 50 h for HER, meeting a key requirement for commercial viability. The superior electrochemical characteristics of the NCP@FSSM electrode are attributed to its low overpotential, high electrochemical surface area (ECSA), high turnover frequency (TOF) number, good faradic efficiency, and elevated mass activity, which drives efficient electrocatalysis. Additionally, the unique binder-free nanorod architecture ensures a dense distribution of active sites and a synergistic interaction among nickel, cobalt, and phosphorus, optimizing both HER and OER performance. The abovementioned results designate that the NCP@FSSM electrode is a promising bifunctional electrode for the water-splitting application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


