一种使壳聚糖化学选择性修饰的替代胺保护策略
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
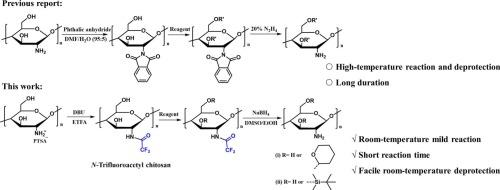
三氟乙酰基是一种应用广泛且易于去除的氨基保护基团,是壳聚糖氨基保护的理想选择。然而,目前壳聚糖的三氟乙酰化方法存在反应效率低、修饰程度有限的问题,这对实现完全的n -三氟乙酰化是一个持续的挑战。本研究提出了一种简单有效的制备氨基保护壳聚糖,特别是n -三氟乙酰壳聚糖的方法。该工艺在室温下均相溶液中进行,不需要严格的无水或无氧条件,反应时间短。n -三氟乙酰壳聚糖在有机溶剂中具有良好的溶解性,可与烯醇醚或t-丁基二甲基氯硅烷等试剂发生均相反应。此外,在温和的条件下,保护基团可以很容易地去除。这种保护壳聚糖氨基的方法为后续修饰提供了方便。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An alternative amine protection strategy for chitosan enabling chemoselective modifications
The trifluoroacetyl group is a widely used and easily removable amino-protecting group, making it an ideal choice for protecting the amino groups of chitosan. However, current methods for trifluoroacetylation of chitosan suffer from low reaction efficiency and limited modification degrees, posing a persistent challenge for achieving complete N-trifluoroacetylation. In this study, a simple and efficient method for preparing amino-protected chitosan, specifically N-trifluoroacetyl chitosan, is presented. The process is carried out in a homogeneous solution at room temperature, without the need for stringent anhydrous or oxygen-free conditions, and involves a brief reaction time. N-trifluoroacetyl chitosan exhibits good solubility in organic solvents, enabling homogeneous reactions with reagents such as enol ethers or t-butyldimethylchlorosilane. Furthermore, the protecting group can be easily removed under mild conditions. This method for amino group protection in chitosan offers a convenient approach for subsequent modifications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


