高效降解有机染料的CeO2/Bi2O3异质结光催化剂的构建
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
解决了传统Fenton法处理成本高和生物法降解周期长的技术限制。开发了新型CeO2/Bi2O3异质结光催化剂,并阐明了ii型异质结通过界面电荷转移抑制电子-空穴复合的光催化机理。高温热解法制备的CeO2/Bi2O3微球(粒径200 ~ 300 nm)比表面积为23.98 m2/g,平均孔径为16.47 nm。对四环素(TC)和亚甲基蓝(MB)的降解效率分别达到79.93%和89.81%,显著高于纯CeO2和Bi2O3。增强的光催化性能源于ii型异质结能带对准和界面电荷分离,它们协同抑制了电子-空穴复合。液相色谱-质谱(LC-MS)和自由基捕获实验阐明了MB染料的光催化降解机理。生物毒性试验表明,降解产物的毒性显著降低,矿化过程产生了对环境无害的矿化产物。CeO2/Bi2O3异质结具有良好的光电活性,光电流密度为253 μA/cm2,开路光电压为- 0.04 V。这些增强是由于其强可见光吸收和低界面电荷转移阻力。本研究为高效低成本处理有机污染物提供了一条有前景的途径,为异质结光催化剂的设计提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
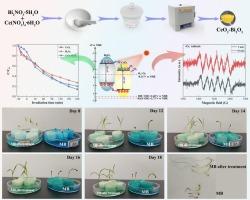
Construction of CeO2/Bi2O3 heterojunction photocatalyst for the efficient degradation of organic dyes
The technological limitations associated with both the substantial treatment costs of the traditional Fenton method and the prolonged degradation cycles of biological methods were addressed. Novel CeO2/Bi2O3 heterojunction photocatalysts were developed, and the photocatalytic mechanism by which the Type-II heterojunctions suppress electron-hole recombination through interfacial charge transfer was elucidated. The CeO2/Bi2O3 microspheres (particle size: 200–300 nm), synthesized via high-temperature pyrolysis, exhibited a specific surface area of 23.98 m2/g and an average pore size of 16.47 nm. The degradation efficiencies for tetracycline (TC) and methylene blue (MB) reached 79.93 % and 89.81 %, respectively, which were significantly higher than those of pure CeO2 and Bi2O3. The enhanced photocatalytic performance originates from the Type-II heterojunction energy band alignment and interfacial charge separation, which synergistically suppress electron-hole recombination. Liquid chromatography-mass spectrometry (LC-MS) and radical trapping experiments elucidated the photocatalytic degradation mechanism of MB dyes. Biotoxicity tests showed significantly reduced toxicity of degradation products, and the mineralization process yielded environmentally benign mineralized products. Furthermore, the CeO2/Bi2O3 heterojunction exhibited remarkable photoelectric activity, achieving a photocurrent density of 253 μA/cm2 and an open-circuit photovoltage of −0.04 V. These enhancements were attributed to its strong visible-light absorption and low interfacial charge-transfer resistance. This study provides a promising approach for efficient and low-cost treatment of organic pollutants, offering new insights into the design of heterojunction photocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


