从实验探索到机理洞察:离子液体对甲醇-乙腈共沸物的分离机理
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
甲醇-乙腈共沸物的高效分离在生物燃料净化和石油化工领域具有重要意义。本研究利用COSMOthermX软件计算了168种离子液体对甲醇和乙腈共沸物分离的选择性。随后,通过萃取精馏确定了[EMIM][OAc]和[N2,2,2,2][OAc]为最适合纯化甲醇和乙腈共沸物的il。在101.3 kPa下测定了甲醇+乙腈+ [EMIM][OAc]/[N2,2,2,2][OAc]的气液平衡数据。il的加入可以提高乙腈对甲醇的相对挥发性。il在特定浓度下能有效消除共沸现象,其分离能力为[N2,2,2,2][OAc] > [EMIM][OAc]。对NRTL模型进行了实验拟合,结果表明该模型具有良好的拟合性能。然后利用σ-分布、多余焓和rdf分析了il共沸分离的机理。结果表明,il更容易与甲醇相互作用,有利于乙腈的提取。[N2,2,2,2][OAc]的提取效果优于[EMIM][OAc],与实验结论一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
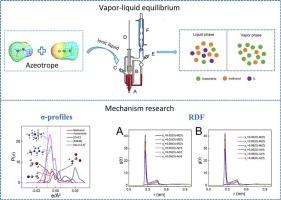
From experimental exploration to mechanistic insight: Separation mechanism of ionic liquids for methanol-acetonitrile azeotrope
The efficient separation of methanol-acetonitrile azeotrope is critical in biofuel purification and petrochemical industries. This study utilized COSMOthermX software to calculate the selectivity of 168 ionic liquids for the separation of methanol and acetonitrile azeotrope. Subsequently, [EMIM][OAc] and [N2,2,2,2][OAc] were identified as the most suitable ILs for the purification of methanol and acetonitrile azeotrope through extractive distillation. The vapor-liquid equilibrium data of methanol + acetonitrile + [EMIM][OAc]/[N2,2,2,2][OAc] were measured at 101.3 kPa. With the addition of ILs, the relative volatility of acetonitrile to methanol can be improved. ILs can effectively eliminate azeotropic phenomena at specific concentrations, with a separation ability of [N2,2,2,2][OAc] > [EMIM][OAc]. The NRTL model was experimentally fitted and found to have good fitting performance. Then, σ-profiles, excess enthalpies, and RDFs were employed to investigate the mechanism of azeotropic separation by ILs. The results revealed that the ILs interact more readily with methanol, facilitating the extraction of acetonitrile. The extraction performance of [N2,2,2,2][OAc] was superior to [EMIM][OAc], consistent with the experimental conclusions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


