一个可逆的化学酶标记策略分析蛋白质o -糖基化
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
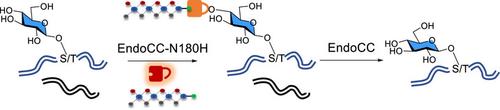
o -连接葡萄糖(O-Glc),丝氨酸残基的β-连接修饰,是一种罕见的蛋白质糖基化形式,首先在含有表皮生长因子(EGF)样结构域(典型O-Glc)的蛋白质上发现。最近的几项研究表明,缺乏egf样结构域的蛋白质也可能经历O-Glc修饰(非规范O-Glc)。然而,由于缺乏有效的分析工具,蛋白质o -糖基化的生物合成起源和生物学功能仍然知之甚少和存在争议。在这里,描述了一种可逆的化学酶标记策略用于O-Glc分析。通过所描述的策略,在人类细胞系中发现了大量规范和非规范的O-Glc位点,表明O-Glc蛋白是一种广泛存在的翻译后蛋白修饰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Reversible Chemoenzymatic Labeling Strategy for Profiling of Protein O-Glucosylation
O-linked glucose (O-Glc), the β-linked modification of serine residues, is a rare form of protein glycosylation first identified on proteins containing epidermal growth factor (EGF)-like domains (canonical O-Glc). Several recent studies revealed that proteins lacking EGF-like domains could also undergo O-Glc modification (noncanonical O-Glc). However, the biosynthetic origin and biological function of protein O-glucosylation remain poorly understood and debated, owing to the lack of effective analytical tools. Here, a reversible chemoenzymatic labeling strategy for O-Glc analysis is described. By the strategy described, a large number of canonical and noncanonical O-Glc sites were identified in human cell lines, indicating that protein O-Glc is a widespread post-translational protein modification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


