通过e型氰基丙烯酸中间体合成利扎布替尼的替代路线
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这篇手稿中,描述了一种通过酰胺形成合成利扎布替尼的替代路线。酰胺形成的两个片段是吡唑嘧啶-哌啶20和e-氰丙烯酸21。Mitsunobu反应的20的产率比现有路线提高了一倍,并且knoevenagel衍生的21具有独占的e构型。与赛诺菲的药物化学路线相比,该路线避免了Z/E异构体分离,总体产量提高了20倍,并通过无过渡金属催化和无色谱中间体纯化提高了可持续性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
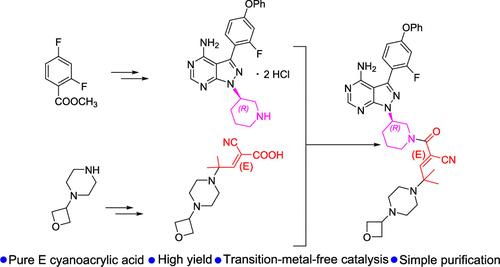
An Alternative Synthetic Route to Rilzabrutinib via an E-Configured Cyanoacrylic Acid Intermediate
In this manuscript, an alternative route of synthesis for making rilzabrutinib via amide formation was described. The two segments for the amide formation are pyrazolopyrimidine-piperidine 20 and E-cyanoacrylic acid 21. The yield of 20 from Mitsunobu reaction was doubled compared with the existing route, and the Knoevenagel-derived 21 exhibited exclusive E-configuration. This route, in contrast to Sanofi’s medicinal chemistry route, avoids Z/E isomer separation, achieves a 20-fold increase in overall yield, and improves sustainability through transition-metal-free catalysis and chromatography-free intermediates purification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


