氨基催化1,6加成2-苄基-3-呋喃醛至3-氰基-4-苯乙烯香豆素:呋喃-香豆素杂化物的脱芳香合成方法
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在手稿中,描述了在氨基催化条件下在1,6加成途径中2-苄基-3-呋喃醛临时脱芳化的应用。在这种反应装置中,由杂芳醛催化生成的二胺与香豆素衍生物1,6加成反应。所开发的方法利用了由氨基催化剂和酸性助催化剂组成的催化体系,这对反应效率至关重要。该反应显示出广泛的底物范围,生成含有呋喃和香豆素基团的目标产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
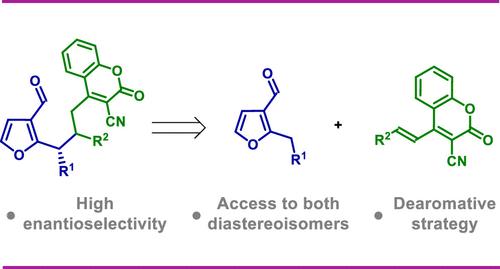
Aminocatalytic 1,6-Addition of 2-Benzyl-3-furaldehyde to 3-Cyano-4-styrylcoumarins: A Dearomative Approach for the Synthesis of Furan–Coumarin Hybrids
In the manuscript, the application of temporary dearomatization of 2-benzyl-3-furaldehyde under aminocatalytic conditions in a 1,6-addition pathway is described. In such reaction setup catalytically generated dienamine derived from heteroaromatic aldehydes reacts with coumarin derivatives in 1,6-addition. Developed approach utilizes a catalytic system consisting of an aminocatalyst and an acidic cocatalyst, which is crucial for the reaction efficiency. The reaction displays a wide substrate scope generating target products containing furan and coumarin moieties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


