利用1,4,5,6-四氢吡啶基脒取代磺酰类似物的外周CB1拮抗作用治疗代谢紊乱
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
外周作用的CB1受体拮抗剂有望治疗肥胖、糖尿病和纤维化疾病,这突出了它们在治疗代谢综合征疾病(MetS)中的重要性。我们通过将脒片段整合到1,4,5,6-四氢吡啶基磺酰脲阻断CB1受体的支架中,合成并评价了化合物。最初的合成包括化合物10a-u,随后扩展到不同的脒基团(11a-18e),以获得详细的构效关系。该化合物具有较高的CB1R结合亲和力,有效的CB1R拮抗剂活性,并具有体外iNOS抑制作用。所选化合物表现出良好的口服暴露,化合物11jE2的脑外显率低于13%,证明存在外周限制。四臂拮抗剂11jE2的体内研究显示,饮食性肥胖(DIO)小鼠的食物摄入量减少,体重减轻。分子对接和模拟分析阐明了结合机制,突出了中央核心的前所未有的椅船构象,可能控制了这一系列化合物的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
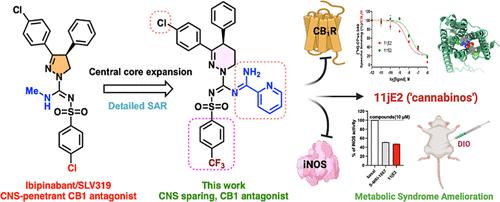
Leveraging Peripheral CB1 Antagonism in 1,4,5,6-Tetrahydropyridazine-Based Amidine Substituted Sulfonyl Analogs for Treating Metabolic Disorders
CB1 receptor antagonists that are peripherally acting hold promise for treating obesity, diabetes and fibrotic disorders highlighting their importance in treating metabolic syndrome disorders (MetS). We synthesized and evaluated compounds by integrating amidine fragments into 1,4,5,6-tetrahydropyridazine-based sulfonyl urea scaffolds for blocking CB1 receptors. Initial synthesis included compounds 10a–u, followed by expansion into distinct amidine groups (11a–18e) for detailed structure–activity relationships. The compounds displayed high CB1R binding affinity, potent CB1R antagonist activities and showed in vitro iNOS inhibition. Select compounds showed good oral exposure, with compound 11jE2 showing less than <13% brain penetrance, attesting to peripheral restriction. In vivo studies of four-arm antagonist 11jE2 revealed decreased food intake and body weight reduction in diet-induced obese (DIO) mice. Molecular docking and simulation analyses elucidated binding mechanisms, highlighting an unprecedented chair-boat conformation of the central core that may govern the actions in this series of compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


