MAO分子表兄弟的作用机制
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烷基硼酸铝(AAB)盐{[iBu2(DMA)Al]2(μ-H)}+[B(C6F5)4]−(AlHAl_DMA; DMA = N,N-二甲基苯胺)能充分活化烯烃聚合的二氯预催化剂,并能作为杂质清除剂,是已建立的甲基铝氧烷(MAO)的分子表兄弟。对于MAO,它具有明确定义的分子结构的优势,本文利用这一点来研究其作为助催化剂的作用机制。特别地,研究了预催化剂(Me2SiCp2)ZrCl2与AlHAl_DMA和稳定的[AliBu2(L)]+的反应,模拟了假定的萃取物[AliBu2(DMA)]+。后一反应分离出一种罕见的单桥Zr - (μ-Cl) - al杂双核加合物(2),这是一种可能从预催化剂中提取氯的中间体。二异丁基氢化铝(DIBAL-H)在2上加成,得到了多个多核Zr/Al加合物,并桥接了μ-Cl和μ-H片段(3-6)。在(Me2SiCp2)ZrCl2和AlHAl_DMA之间的反应中观察到类似的产物,强化了它们是氯/氢化物交换的中间体的假设,这种交换产生了聚合活性的Zr-H物质。用单晶x射线衍射测定了[(Me2SiCp2)Zr]2(μ-H)(μ-Cl)(μ2 -iBu2AlH2)(5)的固态结构。在AlHAl_DMA中μ-H片段的存在似乎也与确定该助催化剂优异的杂质清除性能有关,因为当该助催化剂的溶液暴露于大气中的氧气和水分时,发现μ-H片段的反应速度比Al-iBu部分更快。本文章由计算机程序翻译,如有差异,请以英文原文为准。
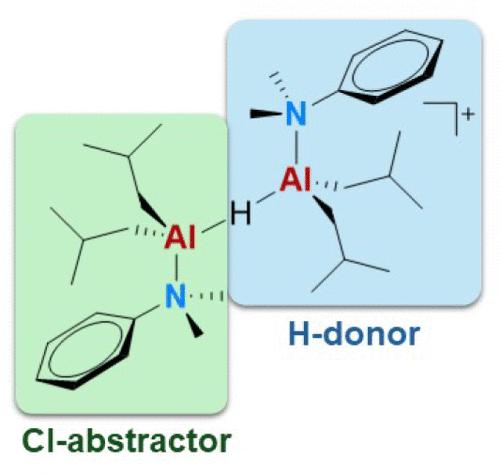
Mechanism of Action of MAO’s Molecular Cousin
The aluminum-alkyl borate (AAB) salt {[iBu2(DMA)Al]2(μ-H)}+[B(C6F5)4]− (AlHAl_DMA; DMA = N,N-dimethylaniline) is able of fully activating dichloride precatalysts for olefin polymerization and serving as an impurity scavenger, thus deserving to be called a molecular cousin of the well-established methylaluminoxane (MAO). With respect to MAO, it offers the advantage of having a well-defined molecular structure, which was exploited herein to investigate its mechanism of action as a cocatalyst. Particularly, the reaction of the precatalyst (Me2SiCp2)ZrCl2 with AlHAl_DMA and with stable [AliBu2(L)]+, modeling the putative abstracting species [AliBu2(DMA)]+, was studied. The latter reaction led to the isolation of a rare, singly bridged Zr–(μ-Cl)–Al heterodinuclear adduct (2), which is a plausible intermediate of chloride abstraction from the precatalyst. Addition of di-iso-butylaluminum hydride (DIBAL-H) to 2 yielded a mixture of several multinuclear Zr/Al adducts with bridging μ-Cl and μ-H fragments (3–6), which were fully characterized by in-depth 2D NMR spectroscopy. Analogous products were observed in the reaction between (Me2SiCp2)ZrCl2 and AlHAl_DMA, reinforcing the hypothesis that they are intermediates of chloride/hydride exchange, which generates a polymerization-active Zr–H species. The solid-state structure of [(Me2SiCp2)Zr]2(μ-H)(μ-Cl)(μ2 -iBu2AlH2) (5) was determined by single-crystal X-ray diffraction. The presence of the μ-H fragment in AlHAl_DMA appears to be relevant also for determining the excellent impurity scavenging properties of this cocatalyst, as it was found to react more rapidly than Al–iBu moieties upon exposure of solutions of this cocatalyst to atmospheric oxygen and moisture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


