胆汁盐和二十六烷基二甲基溴化铵混合体系中的协同胶束作用和囊泡形成
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
可调自组装系统的设计对于生物医学中先进纳米结构的发展越来越重要。单一表面活性剂组合的稳定性和适应性通常有限,而混合表面活性剂体系,特别是那些将生物相关的胆盐与双尾阳离子表面活性剂结合在一起的体系,可以提供更好的物理化学性能。然而,对它们的胶束相互作用、热力学稳定性和聚集形态的综合实验研究仍然有限。本研究系统地研究了胆酸钠(NaC)和脱氧胆酸钠(NaDC)与二十六进基二甲基溴化铵(DHDAB)在水溶液中的自组装行为。使用Clint, Rubingh和Rosen模型分析了不同摩尔分数的表面张力和电导率测量,以确定临界胶束浓度(CMC),表面过量浓度(Γmax),最小分子面积(Amin)和相互作用参数(βm, βo),以及胶束化的热力学特性。负相互作用参数值和持续低cmc在所有成分证实了两亲性成分之间的强协同相互作用。胶束的热力学稳定性受静电互补性、疏水链差、分子间烃类相互作用和离子偶极子力的影响。利用动态光散射(DLS)、小角中子散射(SANS)和透射电子显微镜(TEM)进行的互补结构分析揭示了囊泡胶束的形成,与填料参数和协同自组装的预测一致。热力学分析进一步证实胶束化是自发的,主要由熵驱动。总之,这些发现表明,胆盐-阳离子表面活性剂混合物可以产生稳定和可调的囊泡组件,为纳米载体的开发、药物的控制释放和可持续的材料配方提供了分子框架,同时强调了其生物相容性和靶向递送功能的进一步研究的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
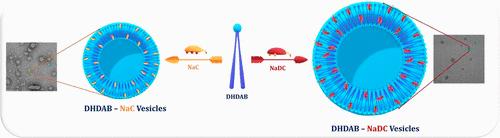
Synergistic Micellization and Vesicle Formation in Bile Salts and Dihexadecyldimethylammonium Bromide Mixed Systems
The design of tunable self-assembly systems is increasingly important for the development of advanced nanostructures in biomedical applications. Single-surfactant assemblies often suffer from limited stability and adaptability, whereas mixed surfactant systems, particularly those combining biologically relevant bile salts with double-tailed cationic surfactants, offer enhanced physicochemical properties. However, comprehensive experimental investigations into their micellar interactions, thermodynamic stability, and aggregation morphologies remain limited. In this study, the self-assembly behavior of sodium cholate (NaC) and sodium deoxycholate (NaDC) with dihexadecyldimethylammonium bromide (DHDAB) in aqueous solution was systematically examined. Surface tension and conductivity measurements across varying mole fractions were analyzed using the Clint, Rubingh, and Rosen models to determine the critical micelle concentration (CMC), surface excess concentration (Γmax), minimum molecular area (Amin), and interaction parameters (βm, βo), along with the thermodynamic characteristics of micellization. Negative interaction parameter values and consistently low CMCs across all compositions confirmed strong synergistic interactions between the amphiphilic constituents. The thermodynamic stability of micellization is governed by electrostatic complementarity, hydrophobic chain disparity, intermolecular hydrocarbon interactions, and ion–dipole forces. Complementary structural analyses using dynamic light scattering (DLS), small-angle neutron scattering (SANS), and transmission electron microscopy (TEM) revealed the formation of vesicular micelles, consistent with predictions of the packing parameter and cooperative self-assembly. Thermodynamic analyses further confirmed that micellization is spontaneous and predominantly driven by entropy. Overall, these findings demonstrate that bile salt–cationic surfactant mixtures can generate stable and tunable vesicular assemblies, providing a molecular framework for nanocarrier development, controlled drug release, and sustainable material formulations, while emphasizing the need for further studies on their biocompatibility and functionalization for targeted delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


