羰基化合物强化电化学加氢的简化方法
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
在本研究中,开发了一种多种羰基化合物的电化学加氢方法,特别强调工业适用性和可扩展性。系统研究了电流密度、溶剂、溶剂比、电解质、质子浓度、电极材料和结构以及底物浓度等参数对收率和选择性的影响。异佛尔酮作为模型化合物,促进了相应的烯酮向酮的过渡。此外,该系统的适用性扩大到包括其他烯酮的加氢,如甲基丙烯酸酯、酮和醛。该方法采用泡沫镍电极和水:甲醇(1:1)与0.5 M硫酸的简单电解质混合物。值得注意的是,使用的电流密度相对较高,为90 mA/cm²,增强了该工艺的工业吸引力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
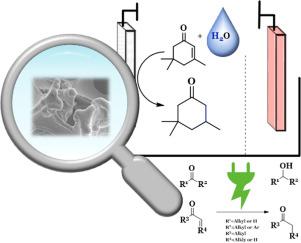
Simplified Approach for an enhanced Electrochemical Hydrogenation of Carbonyl Compounds
In this study, a method for the electrochemical hydrogenation of various carbonyl compounds was developed, with a particular emphasis on industrial applicability and scalability. Each parameter—including current density, solvent, solvent ratio, electrolyte, proton concentration, electrode material and structure, as well as substrate concentration—was systematically investigated for its impact on yield and selectivity. Isophorone served as a model compound, facilitating the transition from the corresponding enone to the ketone. Additionally, the applicability of the system was expanded to include the hydrogenation of other enones such as methacrylates, ketones, and aldehydes. The method employs nickel foam electrodes and a simple electrolyte mixture of water:methanol (1:1) with 0.5 M sulfuric acid. Notably, the current density used is relatively high at 90 mA/cm², enhancing the industrial attractiveness of the process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


