铈掺杂诱导Ni5P4原位重构,增强尿素助水分解
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
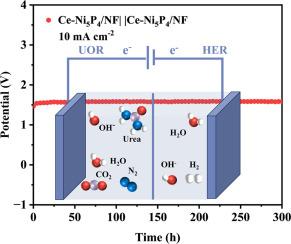
用尿素氧化反应(UOR)代替全水分解的阳极析氧反应(OER),可以显著降低电解制氢的能耗。Ni5P4以其类似贵金属的性质在UOR中具有较高的催化活性,但仍存在活性与稳定性难以结合、双功能催化性能不足的问题。Ce以其独特的电子构型和可变价态(Ce³+ /Ce⁴+)可以调节Ni5P4的电子结构,优化反应中间体的吸附能,从而同时增强催化活性和稳定性。本文采用水热法和磷化法制备了ce掺杂Ni5P4 (Ce-Ni5P4/NF)双功能电催化剂。测试结果表明,在10和100 mA cm-2条件下,尿素氧化反应电位分别为1.331 V和1.412 V, 10 mA cm-2条件下的析氢反应仅需83 mV过电位,总水解性能稳定300小时。原位拉曼进一步证明,Ce可以加速Ni5P4重构为高活性NiOOH,增加活性位点,优化中间吸附,提高效率。本研究为开发先进的双功能电催化剂提供了一条新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cerium doping induces in-situ reconstruction of Ni5P4 to enhance urea-assisted water splitting
Replacing the anodic oxygen evolution reaction (OER) of overall water splitting with urea oxidation reaction (UOR) can significantly reduce the energy consumption of hydrogen production via electrolysis. Ni5P4 has high catalytic activity in UOR with its noble-metal-like properties, but it still has the problems of difficulty in combining activity and stability and insufficient bifunctional catalytic performance. Ce, with its unique electronic configuration and variable valence states (Ce³⁺/Ce⁴⁺), can modulate the electronic structure of Ni5P4 and optimize the adsorption energy of reaction intermediates, thereby enhancing both catalytic activity and stability simultaneously. In this paper, Ce-doped Ni5P4 (Ce-Ni5P4/NF) bifunctional electrocatalysts were prepared by a facile hydrothermal and phosphorization method. Testing results indicated that the potentials of urea oxidation reaction at 10 and 100 mA cm-2 were 1.331 V and 1.412 V, respectively, and hydrogen evolution reaction (HER) up to 10 mA cm-2 required a mere 83 mV overpotential, and the performance of the total hydrolysis was stable for 300 hours. In situ Raman further demonstrated that Ce could accelerate the reconstruction of Ni5P4 to highly active NiOOH, increase the active sites, optimize the intermediate adsorption, and enhance the efficiency. This research presents a novel approach for developing advanced bifunctional electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


