熔融盐驱动的镁石衍生MgO基位演化用于高容量和持久的中温CO2捕集
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
MgO是由天然水镁石(Mg(OH)2)煅烧得到的,NaNO3用于在300 °C和50 % CO2条件下促进碳捕获。当NaNO3/MgO的质量比为22 %时,初始CO2吸附量达到438.2 mg/g,到第30次循环时仍保持在75 %。熔融的NaNO3填充到MgO的孔隙中,有利于晶粒的生长,使MgO的比表面积从114 m2/g增加到12 m2/g。然而,由于Mg2+-O2−键被熔融的NaNO3解离,介质碱性位增加到8.21 mmol/g,增强了CO2的吸附。前10分钟的初始吸附速度较快,随后的缓慢吸附占CO₂总吸附量的90% %以上。原位表征证实了NaNO3在碳化过程中的作用,它自己提供O2−并溶解MgO。MgCO3的析出相在熔化的NaNO3中形成颗粒,而不是在不透水层中形成,因此MgO的碳化水平提高到40% %。吸附量降低25 %是由于450 °C解吸后残留的碳酸盐。DFT计算表明,吸附能随着纳米3在MgO上的负载而降低,价带最大值越来越接近费米能级。纳米3修饰的MgO碳化后,CO2的π键电子态和衬底的p带中心远离费米能级,显示出稳定的构型。该研究为中温CO2捕获提供了一种有前途的水镁石吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
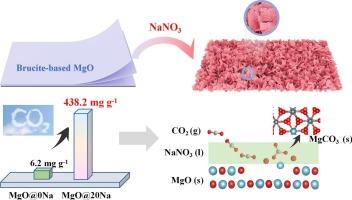
Molten salt-driven basic site evolution in brucite-derived MgO for high-capacity and durable intermediate temperatures CO2 capture
MgO was obtained from the calcination of naturally occurring brucite (Mg(OH)2) and the NaNO3 was used to promote carbon capture at 300 °C under 50 % CO2. As the mass ratio of NaNO3/MgO is 22 %, the CO2 adsorption capacity achieves to 438.2 mg/g initially and remains 75 % at the 30th cycle. The molten NaNO3 fills into the pores of MgO and facilitates the crystallite growth, resulting in the specific surface area from 114 m2/g to 12 m2/g. However, due to the dissociation of Mg2+-O2− bonds by molten NaNO3, the medium basic sites increase to 8.21 mmol/g, enhancing of CO2 adsorption. The initial adsorption in first 10 mins is fast and the subsequent slow adsorption accounts for over 90 % of the total CO₂ adsorption. In situ characterization confirms the contribution of NaNO3 in the carbonation, which provides O2− by itself and dissolves the MgO. Precipitates of MgCO3 grow into particles in melting NaNO3 rather than impermeable layers, so the carbonation level of MgO improves to 40 %. The decrease of 25 % adsorption capacity is ascribed to residual carbonates after desorption at 450 °C. DFT calculations demonstrate that the adsorption energy decreases with the loading of NaNO3 on MgO, as the valence band maximum gets closer to the Fermi level. After the carbonation of NaNO3-modified MgO, the π-bonding electronic state of CO2 and the p-band center of the substrate moves away from the Fermi level, indicating a stable configuration. This study provides a promising brucite-derived adsorbent for medium-temperature CO2 capture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


