I(I)/I(III)催化的二氟化环丙烯重排:二氟烯丙基的区域和立体选择性合成
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二氟烯丙基在制药领域普遍存在,但在构建高取代衍生物方面的合成挑战阻碍了化学空间的探索。因此,继续加紧努力发展显示高度区域选择性和立体选择性的一般办法。为了促进当代有机氟化学这一充满活力的领域,在I(I)/I(III)催化作用下,揭示了密集取代的环丙烯的高效二氟化重排。该平台利用高度直观的开环模型,使二、三、四取代的二氟烯丙基具有高水平的立体选择性,其中瞬态I(III)中心作为一个无迹可循的导向基团。描述了x射线晶体结构分析以及简单的反应后修饰,包括方便地获得氟化索引。鉴于二氟化烯丙基化学型在药物发现中的普遍存在,设想这种操作简单的有机催化平台将加速生物同工体的设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
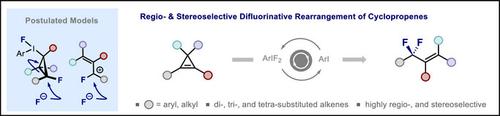
Difluorinative Cyclopropene Rearrangement by I(I)/I(III) Catalysis: Regio- and Stereoselective Synthesis of Allyl Difluorides
Allyl difluorides are pervasive in the pharmaceutical arena, but synthetic challenges in the construction of highly substituted derivatives impede chemical space exploration. Consequently, efforts to develop general approaches that display high levels of regio- and stereo-selectivity continue to be intensively pursued. To contribute to this vibrant area of contemporary organofluorine chemistry, a highly efficient difluorinative rearrangement of densely substituted cyclopropenes is disclosed under the auspices of I(I)/I(III) catalysis. This platform leverages a highly intuitive ring opening model that enables di-, tri-, and tetra-substituted allyl difluorides to be generated with high levels of stereoselectivity where the transient I(III) center serves as a traceless directing group. X-ray crystal structural analysis is described together with facile post-reaction modifications that include expedient access to fluorinated indenes. Given the ubiquity of the allyl difluoride chemotype in drug discovery, it is envisaged that this operationally simple, organocatalytic platform will expedite bioisostere design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


