化学循环反水气转换反应的中试规模研究和反应器模型:共与逆流操作
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
应对气候变化需要能够显著减少工业碳排放,同时促进资源可持续利用的创新方法。本研究在一个kg级中试反应器中使用氧化铁基氧载体研究化学环反水气转移反应(CL-rWGS),比较了在CO2转化率和反应器生产率方面的共流和逆流操作模式,以揭示动力学或热力学限制的程度。实验结果表明,逆流操作始终比并行操作获得更高的平均二氧化碳转化率,在20分钟的持续时间内达到46% %,而在并行模式下为36% %。值得注意的是,逆流配置暂时超过了rWGS反应的气相平衡转化,同时实现了2.9 molCO/kgOC/h的生产率。逆流操作的优越性能源于CO2与Fe2+在Fe0之前的顺序相互作用,从而确保更有效地利用氧载体。此外,降低氧化半循环时间可以提高平均CO2转化率。实验中试反应器数据最好用这样一个模型来描述:(1)平衡约束应用于CO2转化曲线和氧化行为,产生决定转化水平的平衡平台;(2)动力学限制仅影响这些平衡平台之间的过渡速率。反应器模型与逆流实验数据表现出强烈的一致性,而在共流模式中观察到偏差,可能是由于传质限制或未考虑的反应动力学。总的来说,本研究强调逆流操作是优化CL-rWGS的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
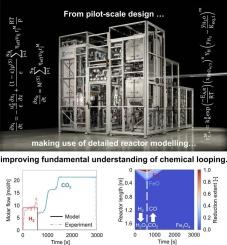
Pilot-scale investigation and reactor modelling of chemical looping reverse water-gas shift reaction: co- versus countercurrent operation
Addressing climate change requires innovative approaches that can significantly reduce industrial carbon emissions while promoting the sustainable use of resources. This study investigates the chemical looping reverse water-gas shift reaction (CL-rWGS) in a kg-scale pilot reactor using iron oxide-based oxygen carriers, comparing cocurrent and countercurrent operational modes in terms of CO2 conversion and reactor productivity, to reveal the extent of kinetic or thermodynamic limitations. Experimental results demonstrate that countercurrent operation consistently achieves higher average CO2 conversions than cocurrent operation, reaching 46 % for a 20-min reduction duration, compared to 36 % in cocurrent mode. Notably, the countercurrent configuration temporarily surpasses the gas-phase equilibrium conversion of the rWGS reaction, while achieving a productivity rate of 2.9 molCO/kgOC/h. The superior performance of countercurrent operation stems from the sequential interaction of CO2 with Fe2+ before Fe0, thereby ensuring more efficient utilization of the oxygen carrier. Additionally, lowering the oxidation half-cycle duration enhances the average CO2 conversion. The experimental pilot reactor data is best described by a model in which (1) equilibrium constraints are applied to CO2 conversion profiles and oxidation behavior, yielding equilibrium plateaus which dictate the conversion levels, and (2) kinetic limitations merely influence the transitioning rate between these equilibrium plateaus. The reactor model exhibits strong agreement with countercurrent experimental data, while deviations are observed in the cocurrent mode, likely due to mass transfer limitations or unaccounted reaction dynamics. Overall, this study highlights countercurrent operation as a promising strategy for optimizing CL-rWGS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


