包裹瑞舒伐他汀的工程损伤归巢脂质体通过阻止泡沫巨噬细胞的形成来增强脊髓损伤修复
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
脊髓损伤后,浸润性巨噬细胞过度吞噬髓鞘碎片,转化为泡沫状巨噬细胞,显著加重继发性损伤病理。在这项研究中,我们开发了半胱氨酸-丙氨酸-谷氨酰胺-赖氨酸(CAQK)四肽修饰脂粒(Lip),包封瑞舒伐他汀(命名为CAQK-Lip- r),专门用于靶向递送脊髓损伤,系统地研究了CAQK-Lip- r介导的抑制泡沫巨噬细胞形成和促进脊髓损伤后功能恢复的治疗效果和分子机制。新设计的CAQK-Lip-R在损伤部位表现出有效的积累,并发挥了实质性的神经保护作用,这可以通过显著促进损伤脊髓的功能恢复来证明。从机制上讲,这些治疗益处是通过增强自噬的开始和进展来调节髓磷脂脂质碎片清除和巨噬细胞极化转化,同时有效抑制PI3K/Akt/mTOR信号轴活性,并随之增加巨噬细胞的自噬通量来实现的。我们的研究结果不仅阐明了CAQK-Lip-R在防止受损脊髓微环境中泡沫巨噬细胞形成中的作用机制,而且还提出了一种利用病变归巢纳米递送系统治疗脊髓损伤的创新治疗模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
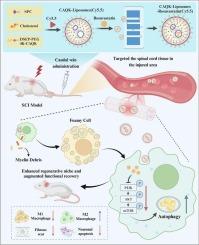
Engineered lesion-homing liposomes encapsulating Rosuvastatin enhance spinal cord injury repair by preventing the formation of foamy macrophages
Following spinal cord injury (SCI), infiltrating macrophages excessively phagocytose myelin debris, subsequently transforming into foamy macrophages that significantly exacerbate secondary injury pathology. In this study, we developed cysteine-alanine-glutamine-lysine (CAQK) tetrapeptide-modified liposomes (Lip) encapsulating Rosuvastatin (designated as CAQK-Lip-R), specifically engineered for targeted delivery to SCI lesions, to systematically investigate both the therapeutic effects and molecular mechanisms underlying CAQK-Lip-R-mediated inhibition of foamy macrophage formation and promotion of functional recovery after spinal cord injury. The novel-designed CAQK-Lip-R demonstrated efficient accumulation at injury sites and exerted substantial neuroprotective effects, as evidenced by significantly promoted functional recovery in the injured spinal cord. Mechanistically, these therapeutic benefits were achieved through regulation of myelin lipid debris clearance and macrophage polarization conversion via enhanced autophagy initiation and progression, coupled with effective suppression of PI3K/Akt/mTOR signaling axis activity and concomitant augmentation of autophagic flux in macrophages. Our findings not only elucidate a previously unrecognized mechanism of CAQK-Lip-R action in preventing foamy macrophage formation within the injured spinal cord microenvironment, but also present an innovative therapeutic paradigm utilizing a lesion-homing nano-delivery system for SCI treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


