与聚乙二醇化相比,脂质体载体的多磷胆碱化可延长血液循环,增强细胞摄取,降低免疫原性
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
静脉脂质体给药在药物疗效方面具有很大的前景,但面临快速清除和免疫系统降解等挑战。基于聚乙二醇的脂质体表面功能化(PEGylation)是目前解决这些问题的金标准和最广泛使用的方法,由于免疫激活,重复给药容易减少细胞摄取和加速血流清除(ABC)效应。我们展示了一种使用聚(2-甲基丙烯酰氧乙基磷酸胆碱)(pMPC)的新型脂质体表面功能化,显着克服了这些限制,同时保持了相当的胶体稳定性。与peg脂质体相比,这种多磷胆碱脂质体表现出可调节的细胞摄取和延长的血液循环时间,两者都受聚合物长度的调节,同时免疫原性降低(IgM抗体激发降低)和ABC效应减弱。这些聚合物长度依赖的特性为优化药物递送系统提供了灵活性,将pMPCylated脂质体定位为具有明显优势的聚乙二醇化制剂的令人信服的替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
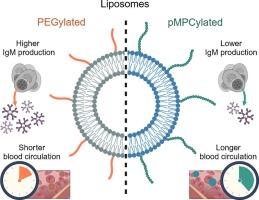
Polyphosphocholination of liposomic vehicles extends blood circulation, enhances cellular uptake, and lowers immunogenicity relative to PEGylation
Intravenous liposomal drug delivery holds great promise for pharmaceutical efficacy, but faces challenges such as rapid clearance and immune system degradation. PEG-based liposome surface functionalization (PEGylation), currently the gold-standard and most widely-used approach to address these issues, is prone to reduced cellular uptake and accelerated bloodstream clearance (ABC) effect upon repeated administration due to immune activation. We demonstrate a novel liposome surface functionalization using poly(2-methacryloyloxyethyl phosphorylcholine) (pMPC) that significantly overcomes these limitations while maintaining comparable colloidal stability. Such polyphosphocholinated liposomes exhibit tunable cellular uptake, and prolonged blood circulation times, both modulated by polymer length, alongside reduced immunogenicity (lower IgM antibody elicitation) and a diminished ABC effect compared to PEG-liposomes. These polymer-length-dependent properties offer flexibility in optimizing drug delivery systems, positioning pMPCylated liposomes as a compelling alternative to PEGylated formulations with clear advantages for liposomal drug delivery therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


