一种安全的基于皂苷的纳米锰介导的STING激活策略
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亲水性治疗剂(例如过渡金属离子和蛋白抗原)的有效递送仍然是免疫治疗中一个重要的药理学障碍。以皂素为基础的输送平台显示出巨大的潜力。使用锰离子(Mn2+)对cGAS-STING通路进行治疗性调节,在癌症免疫治疗中具有重要的前景。然而,除了递送问题外,临床翻译还面临额外的安全性挑战,因为治疗剂量的Mn2+通常会引起慢性炎症和氧化应激。为了解决这些挑战,我们开发了一种仿生纳米颗粒,人血清白蛋白-黄芪甲苷IV- mncl2纳米颗粒(HSA-A-M NPs),利用黄芪甲苷IV (AS-IV)的双重功能。AS-IV通过增加膜通透性来促进Mn2+的摄取,同时抑制NF-κ b驱动的炎症和ROS的产生,提高安全性。纳米颗粒的核心由HSA稳定,提高了生物相容性,而壳聚糖和透明质酸(HA)衍生物(CS-NG@HGS)的表面修饰优化了肿瘤靶向性,产生最终配方CS-NG@HGS @HSA-A-M。体外和体内,该平台增强cgas - sting介导的抗肿瘤免疫,同时最大限度地减少全身Mn2+毒性和炎症。通过将Mn2+的传递与AS-IV的抗炎作用结合起来,它建立了一种“受控激活”模式——增强IFN-I的产生,同时抑制过度的免疫反应。这种双作用设计为金属离子基联合治疗提供了更安全、适应性强的框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
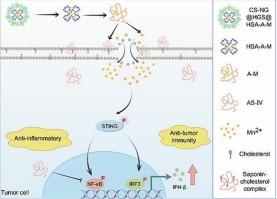
A safe saponin-based nano-strategy for manganese-mediated STING activation
The effective delivery of hydrophilic therapeutic agents (e.g., transition metal ions and protein antigens) remains a significant pharmacological hurdle in immunotherapy. Saponin-based delivery platforms demonstrate remarkable potential. Therapeutic modulation of the cGAS–STING pathway using manganese ions (Mn2+) holds significant promise for cancer immunotherapy. However, clinical translation faces additional safety challenges beyond delivery issues, since therapeutic doses of Mn2+ often induce chronic inflammation and oxidative stress. To address these challenges, we developed a biomimetic nanoparticle, Human serum albumin-Astragaloside IV-MnCl2 nanoparticles(HSA–A–M NPs), leveraging the dual functionality of the saponin astragaloside IV (AS-IV). AS-IV enhances Mn2+ uptake by increasing membrane permeability while suppressing NF-κB-driven inflammation and ROS production, improving safety. The nanoparticle's core, stabilized by HSA, boosts biocompatibility, while surface modifications with chitosan and hyaluronic acid (HA) derivatives (CS-NG@HGS) optimize tumor targeting, yielding the final formulation CS–NG@HGS@HSA–A–M. In vitro and vivo, this platform enhances cGAS–STING-mediated antitumor immunity while minimizing systemic Mn2+ toxicity and inflammation. By combining Mn2+ delivery with AS-IV's anti-inflammatory effects, it establishes a “controlled-activation” paradigm—potentiating IFN-I production yet curbing excessive immune responses. This dual-action design offers a safer, adaptable framework for metal ion-based combination therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


