在PLGA纳米颗粒中远程装载自身抗原治疗多发性硬化症
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
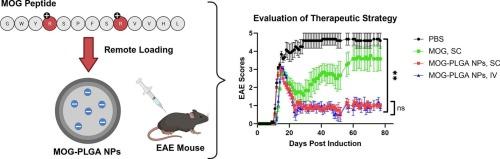
像多发性硬化症(MS)这样的自身免疫性疾病影响着全世界数百万人,并且患病率一直在上升。目前的治疗策略要么完全抑制免疫功能,要么只提供适度的疗效。最近,研究工作已将重点转向抗原特异性治疗,以促进免疫耐受并避免损害一般免疫功能。在这里,我们展示了一种新型的水远程负载方法的应用,该方法使用聚乳酸-羟基乙酸纳米粒子(PLGA NPs)以高负载和高封装效率装载髓鞘少突胶质细胞糖蛋白(MOG)肽。这些NPs(750 ± 200 nm和 − 16.7 ± 0.4 mV zeta电位)在体外缓慢持续释放MOG肽,并降低树突状细胞共刺激分子的表达。单剂量MOG-PLGA NPs给予SC或IV在小鼠实验性自身免疫性脑脊髓炎(EAE) ms模型中表现出很强的疗效。用MOG-PLGA NPs进行预防性治疗可防止疾病进展,而用MOG-PLGA NPs进行治疗性治疗可有效逆转EAE症状。MOG-PLGA NPs也诱导了对EAE再挑战的长期耐受性。在机制上,与PBS或游离MOG肽对照组相比,单次注射MOG- plga NPs诱导MOG特异性CD4+ T调节细胞(Tregs)和无能T细胞的频率增加2倍。此外,组织病理学分析显示脱髓鞘百分比与EAE评分呈正相关。因此,自身抗原,如MOG肽,可以从高负载和EE的水溶液中远程加载到PLGA NPs中,以实现长期控释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Remote loading of an autoantigen in PLGA nanoparticles for the treatment of multiple sclerosis
Autoimmune diseases like multiple sclerosis (MS) affect millions of people worldwide and have been growing in prevalence. Current therapeutic strategies either entirely suppress immune function or only offer modest efficacy. Research efforts have shifted focus more recently to antigen-specific therapies to promote immune tolerance and avoid compromising general immune function. Here, we show the application of a novel aqueous remote loading method using poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) to load myelin oligodendrocyte glycoprotein (MOG) peptide at high loading and encapsulation efficiency. MOG is a target of autoreactive T cells in MS. These NPs (750 ± 200 nm and − 16.7 ± 0.4 mV zeta potential) slowly and continuously released MOG peptide and decreased costimulatory molecule expression on dendritic cells in vitro. A single dose of MOG-PLGA NPs administered either SC or IV exerted strong efficacy in a murine experimental autoimmune encephalomyelitis (EAE) model of MS. Prophylactic treatment with MOG-PLGA NPs prevented disease progression, while therapeutic treatment with MOG-PLGA NPs effectively reversed the EAE symptoms. MOG-PLGA NPs also induced long term tolerance against EAE re-challenge. Mechanistically, a single injection of MOG-PLGA NPs induced a 2-fold increase in the frequency of MOG-specific CD4+ T regulatory cells (Tregs) and anergic T cells, compared with PBS or free MOG peptide control groups. Additionally, histopathological analysis demonstrated a positive correlation between % demyelination and EAE score. Hence, autoantigens, such as MOG peptide, can be remote loaded into PLGA NPs from an aqueous solution at high loading and EE for long-term controlled release.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


