表面工程健壮的混合脂质纳米胶囊:mRNA递送的下一代纳米平台
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
混合脂质纳米胶囊(hnc)是一种很有前途的mRNA递送平台,具有环境温度稳定的额外好处。传统的hnc使用聚乙烯亚胺(PEI)促进mRNA结合、细胞摄取和内体逃逸。然而,PEI的高电荷密度与细胞毒性、非特异性蛋白质结合和先天免疫激活有关,这些特征可能限制其在慢性或重复剂量应用中的效用。为了克服这些限制,我们用l -组氨酸(hLNC- hist)或短链聚乙二醇(hLNC- peg)设计了hLNC表面,以提高生物相容性。这两种修饰都降低了血清蛋白结合并增强了细胞相容性。与未修饰的hnc相比,hnc - hist在体外保存了mRNA转染,并改善了体内表达。相比之下,hnc - peg显著降低了转染效率。值得注意的是,在全身给药后,hnc - hist没有诱导IgM或IgG反应,并抑制循环促炎细胞因子,这与脂质纳米颗粒(LNPs)通常观察到的急性免疫激活和抗peg抗体形成形成相反。尽管在某些模型中,hnc - hist没有达到LNPs的转染效率峰值,但hnc - hist没有表现出限制LNP重复给药的免疫原性和反应原性。hnc - hist为慢性mRNA递送提供了令人信服的替代方案,具有改进的安全性和有效性。重要的是,当嵌入糖玻璃中时,hnc - hist保留了在环境和高温下稳定mRNA的能力,从而实现了潜在的无冷链部署。这些发现表明hnc - hist是一种免疫沉默、生物相容性和热稳定的mRNA递送载体。其重复给药的适用性使其对需要长期给药的新兴mRNA治疗特别有吸引力,包括癌症免疫治疗、蛋白质替代和自身免疫调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
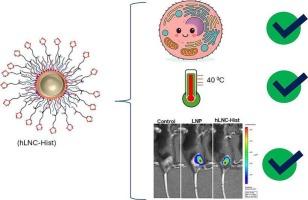
Surface-engineered robust hybrid lipid nanocapsules: A next-generation nanoplatform for mRNA delivery
Hybrid lipid nanocapsules (hLNCs) represent a promising platform for mRNA delivery with the added benefit of ambient-temperature stabilization. Conventional hLNCs use polyethyleneimine (PEI) to facilitate mRNA binding, cellular uptake, and endosomal escape. However, PEI's high charge density is associated with cytotoxicity, nonspecific protein binding, and innate immune activation, features that may limit utility in chronic or repeat-dose applications. To overcome these limitations, we engineered the hLNC surface with either L-histidine (hLNC-Hist) or short-chain polyethylene glycol (hLNC-PEG) to improve biocompatibility. Both modifications reduced serum protein binding and enhanced cytocompatibility. hLNC-Hist preserved mRNA transfection in vitro and improved in vivo expression compared to unmodified hLNCs. In contrast, hLNC-PEG significantly reduced transfection efficiency. Notably, hLNC-Hist did not induce IgM or IgG responses and suppressed circulating pro-inflammatory cytokines following systemic administration - features that stand in contrast to the acute immune activation and anti-PEG antibody formation commonly observed with lipid nanoparticles (LNPs). Although hLNC-Hist did not achieve quite the peak transfection efficiency as LNPs in some models, hLNC-Hist did not exhibit the immunogenicity and reactogenicity that can limit repeated LNP dosing. hLNC-Hist offers a compelling alternative with an improved safety-efficacy profile for chronic mRNA delivery. Importantly, hLNC-Hist retained the ability to stabilize mRNA under ambient and elevated temperatures when embedded in sugar glass, enabling potential cold-chain-free deployment. These findings position hLNC-Hist as an immunologically silent, biocompatible, and thermally stable mRNA delivery vehicle. Its suitability for repeated administration makes it particularly attractive for emerging mRNA therapies requiring chronic dosing, including cancer immunotherapy, protein replacement, and autoimmune modulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


