Polydopamine@Iron/鞣花酸纳米颗粒:自我增强的铁凋亡-细胞凋亡协同作用破坏黑色素瘤治疗的能量代谢
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
黑色素瘤是最具侵袭性的一种皮肤癌,由于其高转移倾向和对传统疗法的耐药性,给治疗带来了重大挑战。为了解决这些限制,我们设计了聚乙二醇(PEG)修饰的纳米平台,通过将金属-多酚网络(由鞣花酸(EA)和亚铁(Fe2+)组成)与聚多巴胺纳米粒子(PDA NPs)集成,称为PDA@Fe2+/EA-PEG (ptfe -PEG) NPs。我们证明了聚四氟乙烯-聚乙二醇NPs具有优异的芬顿样活性、光热转化能力和酪氨酸酶抑制活性。体外研究表明,fe - peg NPs中的外源铁离子通过谷胱甘肽(GSH)耗竭和谷胱甘肽过氧化物酶4 (GPX4)失活诱导铁凋亡,而ea通过其内在药理活性引发caspase介导的细胞凋亡并同时抑制黑色素合成。值得注意的是,PDA NPs不仅介导了近红外照射下的光热消融,还通过产生局部热疗放大了Fe2+/EA网络的解离,从而形成了一个自我强化的治疗循环,增强了铁下沉和细胞凋亡。体内实验证明PFE-PEG NPs对黑色素瘤生长和转移有有效的抑制作用。机制分析进一步表明,PFE-PEG NPs抑制黑色素瘤糖酵解,从而破坏肿瘤微环境中的代谢稳态。这项工作建立了一种多模式的治疗策略,在破坏肿瘤代谢依赖的同时,共同激活铁凋亡和细胞凋亡,从而实现抗黑色素瘤的协同抗肿瘤功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
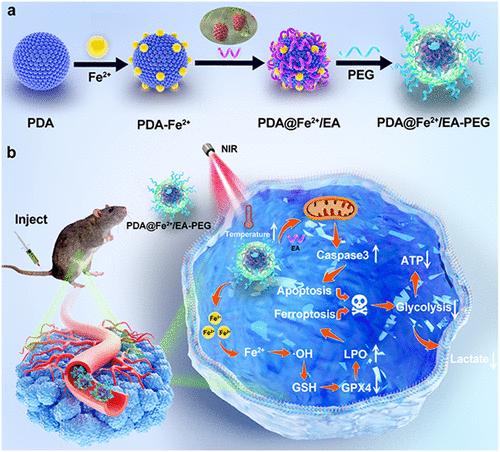
Polydopamine@Iron/Ellagic Acid Nanoparticles: Self-Reinforcing Ferroptosis–Apoptosis Synergy Disrupts Energy Metabolism for Melanoma Therapy
Melanoma, the most aggressive form of skin cancer, presents significant therapeutic challenges due to its high metastatic propensity and resistance to conventional therapies. To address these limitations, we engineered a polyethylene glycol (PEG)-modified nanoplatform by integrating a metal–polyphenol network (composed of ellagic acid (EA) and ferrous iron (Fe2+)) with polydopamine nanoparticles (PDA NPs), termed PDA@Fe2+/EA-PEG (PFE–PEG) NPs. We demonstrated that PFE–PEG NPs exhibited excellent Fenton-like activity, photothermal conversion capability, and tyrosinase inhibitory activity. In vitro studies demonstrated that exogenous iron ions in PFE–PEG NPs induced ferroptosis through glutathione (GSH) depletion and glutathione peroxidase 4 (GPX4) inactivation, whereas EA-triggered caspase-mediated apoptosis and concurrently suppressed melanin synthesis via its intrinsic pharmacological activity. Notably, PDA NPs not only mediated photothermal ablation under NIR irradiation but also amplified Fe2+/EA network dissociation by generating localized hyperthermia, thereby creating a self-reinforcing therapeutic loop that enhanced both ferroptosis and apoptosis. In vivo experiments demonstrated the potent inhibition of melanoma growth and metastasis by PFE–PEG NPs. Mechanistic analysis further revealed that the PFE–PEG NPs suppressed melanoma glycolysis, thereby disrupting metabolic homeostasis in the tumor microenvironment. This work establishes a multimodal therapeutic strategy that coactivates ferroptosis and apoptosis while disrupting tumor metabolic dependencies, thereby achieving synergistic antitumor efficacy against melanoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


