负载表面工程PLGA纳米颗粒的热响应性黏附原位胶凝系统,用于持续阴道内递送依非韦伦用于HIV暴露前预防。
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
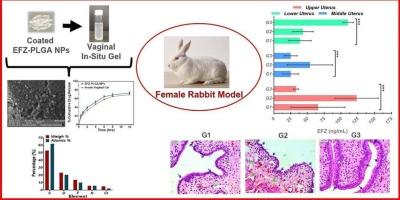
艾滋病毒仍然无法治愈,严重损害免疫系统。本研究提出了一种表面工程的、生物相容性的纳米颗粒凝胶系统,用于HIV PrEP的局部阴道内递送Efavirenz (EFZ)。EFZ是一种BCS II类NNRTI,采用改进的乳液溶剂蒸发方法,采用Box-Behnken设计优化,有效地将EFZ包被Pluronic f -127包被的PLGA纳米颗粒(50:50)(EFZ-PLGA NPs)。流程的优化导致EFZ-PLGA NPs平均大小为144.3 ±2.13 nm,ζ可能性-17.52 ±0.78 mV, PDI 0.248 ± 0.14和87.00的截留效率 ±1.37 %。表面能(87.4 ± 1.17mN/m)表明润湿性增强,有利于生物粘附。AFM和SEM证实了NPs的光滑、离散和球形,FTIR证实了NPs中药物的化学完整性。DSC和PXRD证实了FEZ在聚合物基体中的非晶态。显示的nanoparticle-loaded凝胶溶胶-凝胶转变在38 ±2.2 °C,凝胶在57 ± 2.1秒,13857年粘度 ±172.75 mPa·s(37 ± 0.5°C)和持续释放(69.21 ±2.38 % 12 小时)。MTT试验表明NPs的细胞毒性降低。药物动力学显示显著(P 马克斯:18309 ± 475 ng / mL)比在等离子体(Cmax: 59.7 ± 6 ng / mL),以三倍更高浓度低的子宫(110.41 ±7.98 ng / mL)相比上(33.26 ±3.82 ng / mL)和中等(25.23 ±9.65 ng / mL)子宫,超过的IC50 EFZ(0.442∼-0.82 ng / mL)。组织病理学证实了阴道内制剂的生物相容性和安全性。这项研究表明,凝胶内纳米颗粒平台具有强大的转化潜力,可以为阴道内HIV PrEP提供局部、持续的EFZ递送。该药物递送平台良好的药代动力学、安全性和生物粘附特性突出了其在女性控制的HIV预防策略中的临床进展前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermo-responsive mucoadhesive in-situ gelling system loaded with surface-engineered PLGA nanoparticles for sustained intravaginal delivery of efavirenz in HIV pre-exposure prophylaxis
HIV remains incurable, severely compromising the immune system. This study presents a surface-engineered, biocompatible nanoparticle-in-gel system for localized intravaginal delivery of Efavirenz (EFZ) in HIV PrEP. EFZ, a BCS Class II NNRTI, was efficiently encapsulated into Pluronic F-127 coated PLGA (50:50) nanoparticles (EFZ-PLGA NPs) using a modified emulsion solvent evaporation method optimized employing Box-Behnken design. Optimization of the process resulted in EFZ-PLGA NPs with an average size of 144.3 ± 2.13 nm, ζ-potential of −17.52 ± 0.78 mV, PDI of 0.248 ± 0.14 and entrapment efficiency of 87.00 ± 1.37 %. The surface energy (87.4 ± 1.17mN/m) indicated enhanced wettability, which would favour bioadhesion. AFM and SEM confirmed smooth, discrete and spherical shape of NPs while FTIR confirmed the chemical integrity of drug in NPs. DSC, and PXRD confirmed the amorphous state of FEZ in the polymer matrix. The nanoparticle-loaded gel displayed sol–gel transition at 38 ± 2.2 °C, gelation in 57 ± 2.1sec, viscosity of 13,857 ± 172.75 mPa·s (37 ± 0.5 °C) and sustained release (69.21 ± 2.38 % in 12 hrs). MTT assay indicated the reduced cytotoxicity of NPs. Pharmacokinetics revealed significantly (P < 0.0001) higher vaginal EFZ levels (Cmax: 18,309 ± 475 ng/mL) than that noted in plasma (Cmax: 59.7 ± 6 ng/mL), with 3-fold higher concentration in lower uterus (110.41 ± 7.98 ng/mL) as compared to upper (33.26 ± 3.82 ng/mL) and middle (25.23 ± 9.65 ng/mL) uterus, that exceeded the IC50 of EFZ (∼0.442–0.82 ng/mL). Histopathology confirmed biocompatibility, and safety of intravaginal formulations. This study demonstrates strong translational potential of the nanoparticle-in-gel platform that enables localized, sustained delivery of EFZ for intravaginal HIV PrEP. The favourable pharmacokinetics, safety profile, and bioadhesive properties of the drug delivery platform highlight its promise for clinical advancement in female-controlled HIV prevention strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


