SPINT2抑制nedd4l介导的ACSL4泛素化,促进铁下垂,抑制胆囊癌进展。
IF 8.5
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2025-10-09
DOI:10.1016/j.ijbiomac.2025.148141
引用次数: 0
摘要
胆囊癌(GBC)是一种高度侵袭性的恶性肿瘤,其特点是预后差,治疗选择有限,主要是由于晚期诊断和缺乏明确的分子靶点。该研究首次阐明了SPINT2通过调控铁下垂调控GBC进展的分子机制,并将其与ACSL4稳定性和肿瘤抑制联系起来。尽管SPINT2在多种癌症类型中作为肿瘤抑制因子,但其在GBC中的生物学功能和机制作用仍不明确。在这项研究中,我们发现SPINT2是GBC中铁下沉的关键调节因子。功能实验表明,SPINT2在体外抑制肿瘤细胞的增殖和体内的致瘤性。代谢组学分析显示,SPINT2缺乏改变脂质代谢,降低对铁下垂的易感性。机制上,SPINT2与ACSL4相互作用,通过E3连接酶NEDD4L阻止ACSL4泛素化,从而稳定ACSL4蛋白,促进铁致细胞死亡。临床上,SPINT2低表达与分化差、肿瘤分期晚期、总生存期差有显著相关。总的来说,这些发现将SPINT2定位为GBC中嗜铁途径的关键调节剂,并强调了其作为预后生物标志物和GBC患者嗜铁性干预的治疗切入点的转化前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
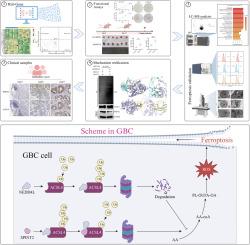
SPINT2 inhibits NEDD4L-mediated ACSL4 ubiquitination to promote ferroptosis and suppress gallbladder cancer progression
Gallbladder cancer (GBC) is a highly aggressive malignancy characterized by poor prognosis and limited therapeutic options, largely due to late-stage diagnosis and a lack of defined molecular targets. This is the first study to elucidate the molecular mechanism by which SPINT2 governs GBC progression through regulation of ferroptosis, linking it to ACSL4 stability and tumor suppression. Although SPINT2 has been implicated as a tumor suppressor in multiple cancer types, its biological function and mechanistic role in GBC have remained elusive. In this study, we identify SPINT2 as a key regulator of ferroptosis in GBC. Functional assays demonstrated that SPINT2 suppresses tumor cell proliferation in vitro and tumorigenicity in vivo. Metabolomic profiling revealed that SPINT2 deficiency alters lipid metabolism and reduces susceptibility to ferroptosis. Mechanistically, SPINT2 interacts with ACSL4 and prevents its ubiquitination by the E3 ligase NEDD4L, thereby stabilizing ACSL4 protein and promoting ferroptotic cell death. Clinically, low SPINT2 expression was significantly associated with poor differentiation, advanced tumor stage, and worse overall survival. Collectively, these findings position SPINT2 as a pivotal modulator of the ferroptotic pathway in GBC and highlight its translational promise as both a prognostic biomarker and a therapeutic entry point for ferroptosis-based interventions in GBC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


