USP21自身抑制和组蛋白H2AK119去泛素化的机制
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
单泛素化组蛋白H2A赖氨酸119 (H2AK119ub)是一种与转录沉默和异染色质形成相关的修饰。泛素特异性蛋白酶21 (USP21)是四种主要的h2ak119特异性去泛素化酶(DUBs)之一,在多种细胞过程中起着关键作用。然而,USP21特异性去泛素化H2AK119ub并受到调控的机制尚不清楚。我们确定了与H2AK119ub核小体结合的USP21催化结构域的低温电镜结构,揭示了与其他h2ak119特异性DUBs不同的识别模式。我们意外地发现USP21的n端IDR抑制了酶的活性。使用alphafold - multitimer对USP21相互作用物进行虚拟筛选,我们确定了磷酸化USP21 IDR的激酶,从而减轻了自身抑制。USP21的AlphaFold3模型提示了一个自抑制的结构模型。AlphaFold分析表明磷酸化调节的自抑制可能是各种USP酶的一个特征。这些发现揭示了H2AK119去泛素化的机制,并揭示了一种以前未被探索的磷酸化依赖性DUB自动调节模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
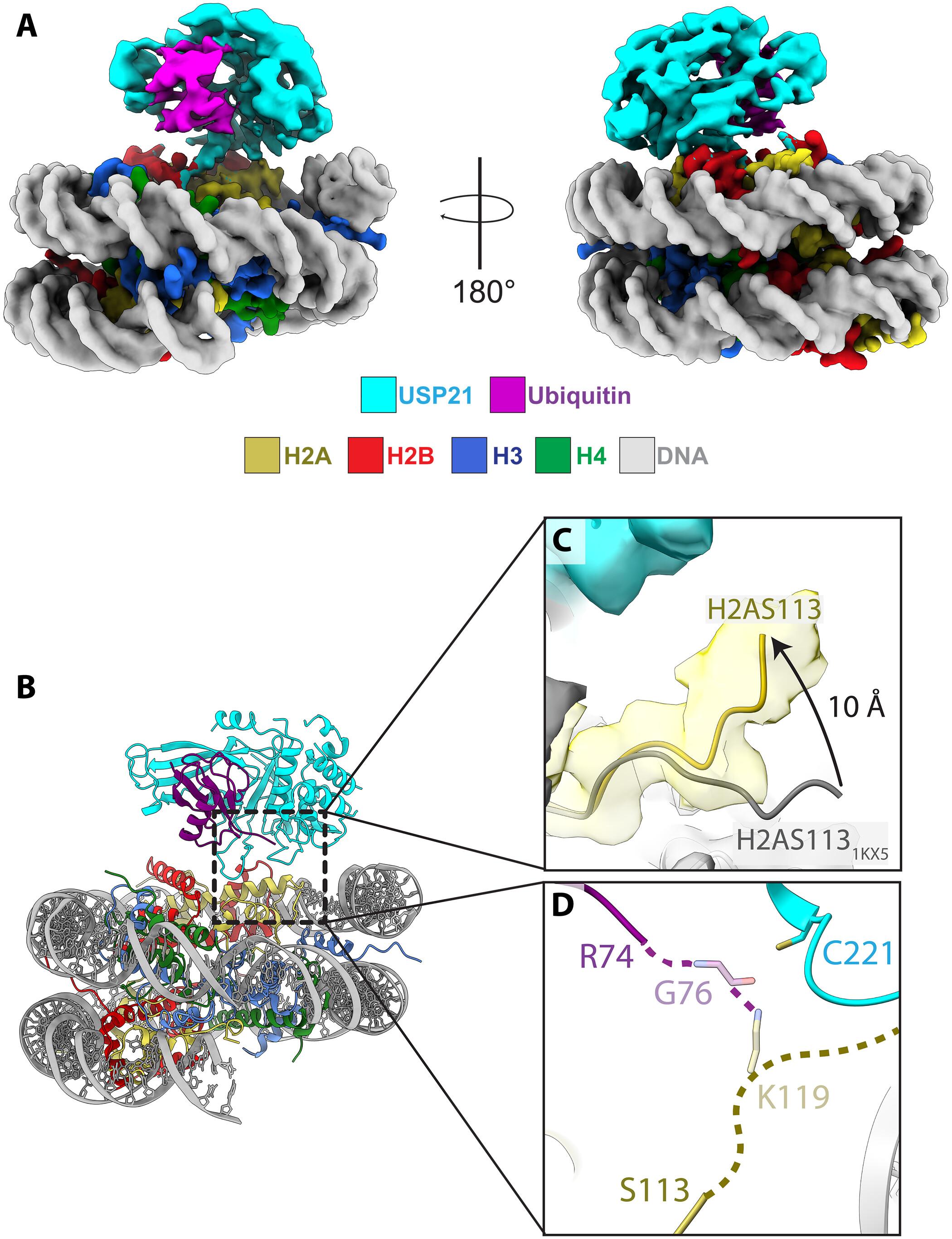
Mechanism of USP21 autoinhibition and histone H2AK119 deubiquitination
Monoubiquitinated histone H2A lysine 119 (H2AK119ub) is a modification associated with transcriptional silencing and heterochromatin formation. Ubiquitin-specific protease 21 (USP21), one of four major H2AK119-specific deubiquitinating enzymes (DUBs), plays critical roles in diverse cellular processes. However, the mechanisms by which USP21 specifically deubiquitinates H2AK119ub and is regulated are unknown. We determined the cryo-EM structure of the USP21 catalytic domain bound to an H2AK119ub nucleosome, which revealed a recognition mode that differs from that of other H2AK119-specific DUBs. We unexpectedly found that the N-terminal IDR of USP21 inhibits the enzyme’s activity. Using AlphaFold-Multimer to perform a virtual screen of USP21 interactors, we identified kinases that phosphorylate the USP21 IDR and thereby relieve autoinhibition. AlphaFold3 modeling of USP21 suggests a structural model for autoinhibition. AlphaFold analysis suggests that phosphorylation-regulated autoinhibition may be a feature of various USP enzymes. These findings shed light on the mechanisms of H2AK119 deubiquitination and reveal a previously unexplored mode of phosphorylation-dependent DUB autoregulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


