MK8722通过激活Sesn2和转录上调BNIP3来促进线粒体自噬,抑制软骨细胞铁凋亡,从而缓解骨关节炎
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
骨关节炎(OA)通常伴有关节软骨的不可逆破坏,难以有效缓解,主要是由于发病机制不清楚和缺乏有效的治疗干预。Sestrin 2 (Sesn2)是一种高度保守的蛋白,调节氧化应激和细胞代谢;然而,其对OA进展的影响以及这一过程的详细机制尚未阐明。目的探讨Sesn2在OA软骨降解中的关键作用,阐明MK8722通过激活Sesn2促进线粒体自噬、抑制软骨细胞铁凋亡的机制。方法我们利用来自人和小鼠模型的多组学数据来研究Sesn2与软骨细胞铁下垂之间的潜在关联。我们通过内侧半月板不稳定手术建立了小鼠OA模型。多种分子生物学技术,包括western blot、免疫荧光和流式细胞术,结合组织学分析,阐明Sesn2在OA进展中的关键作用。结果骨性关节炎关节软骨组织中ssesn2表达降低,Sesn2是调控软骨细胞铁下垂的关键基因。关节内注射腺相关病毒在软骨细胞中过表达Sesn2,通过抑制铁下垂减轻OA软骨损伤。此外,我们发现了一种激活Sesn2 MK8722的药物,通过促进线粒体自噬来抑制软骨细胞衰老和铁下垂,从而减轻软骨破坏。MK8722激活Sesn2,通过转录上调bcl-2相互作用蛋白3 (BNIP3),促进核因子红细胞2相关因子2 (Nrf2)蛋白表达,进而促进线粒体自噬。线粒体自噬的上调随后减少细胞氧化应激和铁下垂,从而减轻OA软骨退变。本研究强调了Sesn2作为一种维持软骨细胞代谢稳态和氧化还原平衡的新蛋白的作用,并表明激活Sesn2的MK8722可能是一种有希望的OA治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
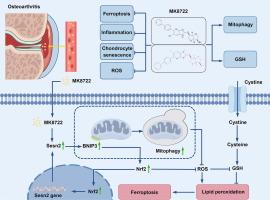
MK8722 alleviates osteoarthritis by activating Sesn2 and transcriptionally upregulating BNIP3 to promote mitophagy and inhibit chondrocyte ferroptosis
Introduction
Osteoarthritis (OA) is commonly accompanied by irreversible destruction of articular cartilage and is difficult to effectively relieve, primarily due to the unclear pathogenesis and the lack of effective therapeutic interventions. Sestrin 2 (Sesn2) is a highly conserved protein that regulates oxidative stress and cellular metabolism; however, its impact on the progression of OA and the detailed mechanisms underlying this process have not been elucidated.Objectives
To investigate the critical role of Sesn2 in OA cartilage degradation and to clarify the underlying mechanism by which MK8722 promotes mitophagy and inhibits chondrocyte ferroptosis through the activation of Sesn2.Methods
We utilized multi-omics data from both human and mouse models to investigate a potential association between Sesn2 and chondrocyte ferroptosis. We established a murine OA model through destabilization of the medial meniscus surgery. Various molecular biological techniques, including western blot, immunofluorescence and flow cytometry, in combination with histological analyses, were employed to elucidate the pivotal role of Sesn2 in the progression of OA.Results
Sesn2 expression is decreased in OA articular cartilage, and Sesn2 is a key gene regulating chondrocyte ferroptosis. Intra-articular injection of adeno-associated virus overexpressed Sesn2 in chondrocytes to alleviate OA cartilage damage by inhibiting ferroptosis. In addition, we identified a drug that activates Sesn2, MK8722, which inhibits chondrocyte senescence and ferroptosis by promoting mitophagy to alleviate cartilage destruction. MK8722 activates Sesn2 and transcriptionally upregulates bcl-2 interacting protein 3 (BNIP3), promoting nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression, and then promoting mitophagy. Upregulation of mitophagy subsequently reduces cellular oxidative stress and ferroptosis, thereby alleviating OA cartilage degeneration.Conclusion
This study underscores the role of Sesn2 as a novel protein that maintains chondrocyte metabolic homeostasis and redox balance, and demonstrates that MK8722, which activates Sesn2, may serve as a promising therapeutic approach for OA.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


