聚七嗪酰亚胺光催化海水高效分裂的机理:抑制离子渗出和改善电荷分离
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
聚七嗪亚胺钾(KPHI)是一种极有前途的光催化剂,通过水裂解裂解氢,特别是在海水中,离子显著提高其活性。然而,这种增强背后的机制仍然存在争议。本研究阐明了海水离子可以抑制K+的渗出,并通过离子交换进一步进入KPHI的框架,增强光生电子-空穴对的实时分离和转移。综合表征表明,电荷分离效率取决于离子类型和浓度。其中,K+浓度为0.2 m时对PHI光催化析氢活性的增强效果最好,密度泛函理论(DFT)模拟表明,碱金属离子的进入产生了更大的静电势和电子转移能力。这些发现阐明了离子介导的光催化性能增强,为优化海水裂解制氢和推进可持续能源技术提供了重要见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
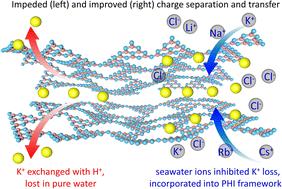
Mechanism insights for efficient photocatalytic seawater splitting in poly(heptazine imide): inhibited ion exudation and improved charge separation
Potassium poly(heptazine imide) (KPHI) has emerged as a highly promising photocatalyst for H2 evolution via water splitting, particularly in seawater, where ions significantly enhance its activity. However, the mechanisms underlying this enhancement remain debated. This study elucidates that seawater ions can inhibit the exudation of K+ and further enter the framework of KPHI through ion exchange, enhancing the real-time separation and transfer of photogenerated electron–hole pairs. Comprehensive characterization reveals that charge separation efficiency depends on both ion type and concentration. Among them, K+ exhibits the best effect on the enhancement of photocatalytic H2 evolution activity of PHI when the concentration is 0.2 M. Density Functional Theory (DFT) simulations show that the entry of alkali metal ions generates a greater electrostatic potential and electron transfer capacity. These findings clarify the ion-mediated enhancement of photocatalytic performance, providing critical insights for optimizing H2 production from seawater splitting and advancing sustainable energy technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


