猪牙周复合体再生中再现原生软硬组织界面的互锁骨/纤维结构
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于缺乏能够概括牙周组织内软硬组织界面的层次互锁结构的功能性材料结构,牙周复合体的修复提出了重大挑战。在这里,通过在其层次结构中设计互锁的骨纤维界面来制造生物启发结构,以模仿天然牙周韧带(PDL) -骨内植的多尺度特征。3d打印的骨模块和静电纺纤维模块提供了合适的结构、生物力学和细胞因子景观,以招募修复细胞并促进其成骨和纤维化分化。在双模块结构的界面处,纤维模块的静电纺纳米纤维由于各种分子间相互作用被插入到骨模块中,这促进了双模块之间的强连接,并沿着插入的纳米纤维提供了生物物理梯度,类似于pdl骨插入的微观结构和微观力学。当构建体放置在猪牙周缺损中12周后,牙槽骨恢复到原来的水平,新形成的成熟胶原纤维插入到再生的牙槽骨中,最终实现软硬组织界面的极好整合。研究结果表明,具有互锁骨纤维界面的生物启发结构可以再生猪体内复杂的软硬组织界面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
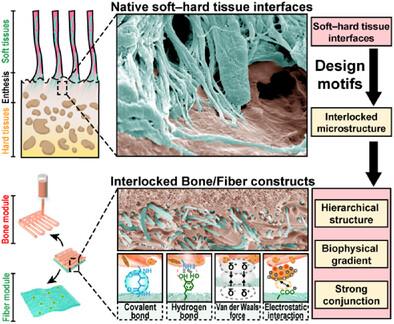
An Interlocked Bone/Fiber Construct Recapitulating the Native Soft‒Hard Tissue Interfaces for Periodontal Complex Regeneration in Pigs
The repair of periodontal complexes poses a significant challenge due to the lack of functional material constructs that can recapitulate the hierarchically interlocked structures of the soft–hard tissue interfaces within periodontium. Here, a bioinspired construct is fabricated by engineering interlocked bone–fiber interfaces within its hierarchical architectures to mimic the multiscale features found in native periodontal ligament (PDL)‒bone enthesis. The 3D-printed bone module and electrospun fiber module of the bioinspired constructs offered suitable architecture, biomechanics, and cytokine landscapes to recruit reparative cells and promote their osteogenic and fibrogenic differentiation. At the interfaces of the dual-module construct, the electrospun nanofibers of the fiber module are inserted into the bone module owing to various intermolecular interactions, which facilitate strong conjunction between the dual modules and provided biophysical gradients along the inserted nanofibers, similar to the microstructure and micromechanics of PDL‒bone insertions. When the constructs are placed in periodontal defects in pigs for 12 weeks, the alveolar bone is restored to its native level, and newly formed mature collagen fibers are inserted into the regenerated alveolar bone, culminating in superb integration between the soft and hard tissue interfaces. The findings demonstrate that bioinspired constructs with interlocked bone–fiber interfaces can regenerate complex soft‒hard tissue interfaces in pigs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


