利用原子精确的Au25纳米团簇揭示葡萄糖在金表面氧化的热催化和电催化途径
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于缺乏对催化剂表面与葡萄糖之间相互作用的理解,电化学氧化葡萄糖的性能被高估了。在此,我们利用原子定义明确的Au25纳米团簇揭示了葡萄糖氧化的机制,在保持结构完整性的同时表现出特殊的活性。氧化反应活性受外加电位和相应改变的Au表面化学状态的显著影响,有三种不同的机制导致不同电位区域的优势:开路附近的有氧氧化途径,低电位下的1e -电催化途径,以及高电位下典型的2e -电催化途径。有氧途径涉及葡萄糖氧化与O2还原的耦合,分别产生葡萄糖酸和H2O2,没有应用潜力。在1e -途径中,葡萄糖的α-氢被Au表面剥离,导致在阳极同时生成葡萄糖酸和H2。揭示这些途径可以精确评估性能,在所有潜在范围内实现100%的法拉第效率。我们的研究结果为阐明生物质电催化的结构-活性关系提供了一个明确的框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
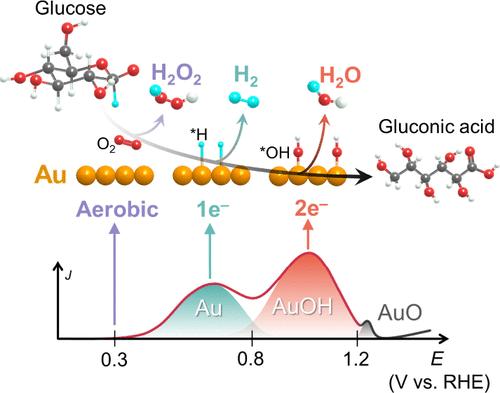
Unraveling the Thermocatalytic and Electrocatalytic Pathways of Glucose Oxidation on Gold Surfaces Using Atomically Precise Au25 Nanoclusters
The performance of electrochemical glucose oxidation has been overestimated due to a lack of understanding of the interactions between the catalyst surface and glucose. Herein, we unravel the mechanisms of glucose oxidation using atomically well-defined Au25 nanoclusters, exhibiting exceptional activity while maintaining structural integrity. The oxidation reactivity is significantly influenced by applied potential and accordingly altered chemical state of the Au surface, with three distinct mechanisms leading to dominance at different potential regions: an aerobic oxidation pathway near open-circuit, a 1e– electrocatalytic pathway prevailing at low potentials, and a typical 2e– electrocatalytic pathway dominating at higher potentials. The aerobic pathway involves coupling glucose oxidation with an O2 reduction, producing gluconic acid and H2O2, respectively, without an applied potential. In the 1e– pathway, the α-hydrogen of glucose is stripped by the Au surface, resulting in a simultaneous production of gluconic acid and H2 at the anode. Unveiling these pathways enabled the precise evaluation of performance as 100% Faraday efficiency across all potential ranges. Our findings provide a definitive framework for elucidating the structure–activity relationship in the electrocatalysis of biomass.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


