双锁纳米光敏剂利用切伦科夫辐射治疗深部肿瘤
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
由于光的穿透深度有限,光动力疗法在深部肿瘤的治疗中面临着巨大的挑战。切伦科夫辐射诱导PDT (CR-PDT)通过利用放射性核素产生的发光提供了一个潜在的解决方案。然而,在常规CR-PDT系统中同时递送放射性核素和光敏剂会导致健康组织不必要的光毒性。在这里,我们提出了一种肿瘤微环境(TME)激活的双锁定纳米光敏剂(89Zr-PHZ-BrCyE),它整合了89zr标记的ph响应聚合物胶束和羧酸酯酶(CrES)激活的光敏剂前药,用于精确的深部肿瘤治疗。双反应CR-PDT系统对肝细胞癌(HepG2)细胞具有高度选择性的细胞毒性,对正常肝细胞的影响最小(L02)。经静脉注射,89Zr-PHZ-BrCyE在皮下和原位H22肿瘤模型中均表现出强大的肿瘤抑制作用和良好的生物安全性。该方法在保证良好生物安全性的同时,有望改善CR-PDT治疗深部肿瘤的效果,为未来潜在的临床应用铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
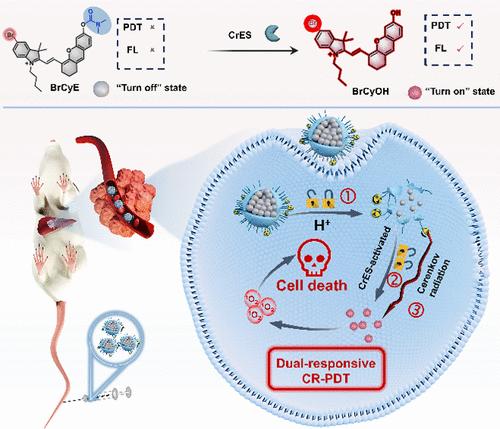
Dual-Locked Nanophotosensitizer Harnessing Cerenkov Radiation for Deep Tumor Therapy
Photodynamic therapy (PDT) faces significant challenges in treating deep tumors due to the limited light penetration depth. Cerenkov radiation-induced PDT (CR-PDT) offers a potential solution by harnessing luminescence derived from radionuclides. However, the simultaneous delivery of radionuclides and photosensitizers in conventional CR-PDT systems leads to unnecessary phototoxicity of healthy tissues. Here, we present a tumor microenvironment (TME)-activated, dual-locked nanophotosensitizer (89Zr-PHZ-BrCyE) that integrates 89Zr-labeled pH-responsive polymeric micelle and a carboxylesterase (CrES)-activated photosensitizer prodrug for precise deep tumor therapy. The dual-responsive CR-PDT system demonstrated highly selective cytotoxicity toward hepatocellular carcinoma (HepG2) cells, with minimal impact on normal liver cells (L02). Upon intravenous injection, 89Zr-PHZ-BrCyE exhibited robust tumor inhibition and excellent biosafety in both subcutaneous and orthotopic H22 tumor models. This approach holds great promise for improving the therapeutic outcomes of CR-PDT in deep-seated tumors while ensuring good biosafety, paving the way for future potential clinical applications in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


