Ce和/或Y修饰BaFeO3 -δ的氧交换动力学:氧同位素交换和压力弛豫
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
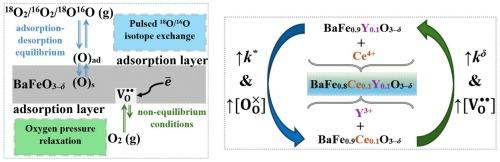
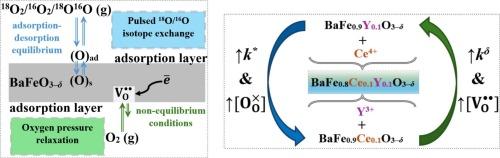
采用两种独立的方法研究了具有三H+/O2 - /e -电导率的掺杂钡铁氧体氧化物电极材料与气相表面氧交换动力学。在ΔT = 600 ~ 800℃,ΔpO2 = 0.1 ~ 3.5 kPa的非平衡条件下,用氧压松弛法测定了bafe0.9 ce0.1 ~ 0.3 -δ、bafe0.9 y0.1 ~ 0.3 -δ和bafe0.8 ce0.1 ~ 0.3 -δ致密样品的氧化学表面交换系数(kδ)。发现了化学表面氧交换系数与氧化物氧含量之间的相关性。采用脉冲同位素交换法(PIE)测定了气态氧-氧化物体系中表面氧交换的机理,在ΔT = 200-600℃,氧气pO2分压 = 21.3 kPa的条件下,在氧化粉末上达到吸附-解吸平衡。计算了氧非均相表面交换速率(rH)和氧交换的基本步骤速率,并确定了这些过程的有效活化能。讨论了温度、氧压和材料阳离子组成对氧交换动力学特性变化的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oxygen exchange kinetics of BaFeO3–δ modified with Ce and/or Y: oxygen isotope exchange and pressure relaxation
The kinetics of surface oxygen exchange between the gas phase and new, highly efficient electrode materials based on doped barium ferrite oxide with triple H+/O2–/e– conductivity were studied by two independent methods. The oxygen chemical surface exchange coefficients (kδ) for BaFe0.9Ce0.1O3–δ, BaFe0.9Y0.1O3–δ and BaFe0.8Ce0.1Y0.1O3–δ dense samples have been measured by oxygen pressure relaxation (OPR) method under non-equilibrium conditions at ΔT = 600–800 °C and ΔpO2 = 0.1–3.5 kPa. A correlation was found between the chemical surface oxygen exchange coefficient and the oxygen content of the oxides. The mechanism of surface oxygen exchange in the gaseous oxygen – oxide system was determined by the method of pulsed isotope exchange (PIE) under conditions of adsorption–desorption equilibrium on oxide powders at ΔT = 350–600 °C and a partial pressure of oxygen pO2 = 21.3 kPa. The values of the rates of oxygen heterogenous surface exchange (rH) and the rates of elementary steps of oxygen exchange were calculated, and the effective activation energies of these processes were determined. The influence of the temperature, the oxygen pressure and the cationic composition of the material on the change in the kinetic characteristics of oxygen exchange was discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


