加巴喷丁内酰胺通过钙-线粒体- ros级联诱导神经发育缺陷
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
加巴喷丁内酰胺(Gabapentin lactam, GBP-L)是抗惊厥药加巴喷丁的一种转化产物,由于其对常规处理工艺的抗性,在地表水中的浓度可达μg/L。尽管人们越来越关注药物转化产品,但它们的机械毒性途径仍然缺乏特征。在这里,我们使用斑马鱼胚胎-幼虫模型来研究环境相关GBP-L浓度(8、80和800 μg/L)对神经发育的影响。暴露于GBP-L对斑马鱼胚胎的存活率没有显著影响,但显著影响了斑马鱼胚胎的孵化过程,增加了畸形率,并抑制了体长发育——在72 hpf时,与对照组相比,体长减少了3.86%,在144 hpf时,体长减少了3.70%。运动评估显示游泳活动的浓度依赖性减少。同时,转基因成像显示神经元荧光强度降低了9.46%,轴突长度减少了7.84%。机制研究表明,GBP-L激活钙信号通路,触发细胞内Ca 2 +过载,导致线粒体功能障碍、活性氧(ROS)产生增加和神经递质稳态破坏。与钙通道阻滞剂维拉帕米共同暴露可显著减轻这些影响,证实钙信号是GBP-L神经毒性的分子启动事件。转录组学分析进一步证实了钙-线粒体- ros级联是关键的毒性途径。该研究首次证明,持久性药物转化产物GBP-L可以通过干扰与环境相关浓度的钙信号来破坏神经发育,这突出了建立包括转化产物在内的综合风险评估框架的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
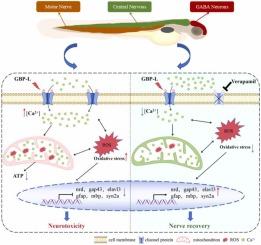
Gabapentin lactam induces neurodevelopmental deficits via calcium-mitochondria-ROS cascade
Gabapentin lactam (GBP-L), a transformation product of the anticonvulsant gabapentin, persists in surface waters at concentrations reaching μg/L levels due to its recalcitrance to conventional treatment processes. Despite growing concerns about pharmaceutical transformation products, their mechanistic toxicity pathways remain poorly characterized. Here, we used zebrafish embryo-larval models to investigate the neurodevelopmental effects of environmentally relevant GBP-L concentrations (8, 80, and 800 μg/L). Exposure to GBP-L had no significant effect on the survival rate of zebrafish embryos, but significantly impaired the hatching process, increased the malformation rate, and inhibited body length development—with a 3.86 % reduction in body length compared to the control group at 72 hpf and a 3.70 % reduction at 144 hpf. Motor assessment revealed a concentration-dependent reduction in swimming activity. Meanwhile, transgenic imaging indicated a decrease in neuronal fluorescence intensity by 9.46 %, alongside a reduction in axonal length of 7.84 %. Mechanistic investigations demonstrated that GBP-L activates calcium signaling pathways, triggering intracellular Ca²⁺ overload that leads to mitochondrial dysfunction, increased reactive oxygen species (ROS) production, and disrupted neurotransmitter homeostasis. Co-exposure with the calcium channel blocker verapamil significantly mitigated these effects, confirming calcium signaling as the molecular initiating event in GBP-L neurotoxicity. Transcriptomic analyses further validated the calcium-mitochondria-ROS cascade as the critical toxicity pathway. This study provides the first evidence that GBP-L, a persistent pharmaceutical transformation product, can disrupt neurodevelopment by perturbing calcium signalling at environmentally relevant concentrations, highlighting the need to establish a comprehensive risk assessment framework that includes transformation products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


