氧化环境中UO2(110)和(111)的表面吸附和溶解机制:对可持续核燃料回收的见解
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二氧化铀(UO2)在潮湿环境中的界面反应对核安全和储存至关重要,但人们对其了解甚少。本研究采用第一性原理DFT + U计算研究了O2和H2O2在UO2(110)和(111)表面的吸附及其对表面溶解行为的影响。结果表明,该材料对O2具有物理和化学吸附作用,同时对H2O2具有解离吸附作用。键长为1.235 Å-1.254 Å的O2的振动频率为1451.0 cm−1 - 1509.5 cm−1,而键长为1.334 Å-1.338 Å的O2的振动频率为1133.6 cm−1 - 1184.1 cm−1,表明非解离的O2表现为超氧化物(O2 -)。为了阐明在潮湿空气或氧化溶液中氧化表面的原子演化,我们进一步探索了H2O分子与最稳定的O2/H2O2吸附构型之间的相互作用。结果表明,H2O解离成OH基团和H原子,形成U-O和H - o键。这项工作为UO2表面上O2/H2O2的吸附形态及其在氧化物界面与水的相互作用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
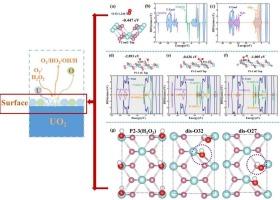
Surface adsorption and dissolution mechanisms of UO2 (110) and (111) in oxidizing environments: Insights for sustainable nuclear fuel recycling
The interfacial reaction of uranium dioxide (UO2) in humid environments is critical for nuclear safety and storage but remains poorly understood. This study employs first-principles DFT + U calculations to investigate O2 and H2O2 adsorption on UO2 (110) and (111) surfaces and their impact on surface dissolution behavior. The results reveal both physical and chemical adsorption of O2, alongside dissociative adsorption of H2O2. The vibrational frequencies of O2 with bond lengths of 1.235 Å–1.254 Å is 1451.0 cm−1–1509.5 cm−1, while O2 with bond lengths of 1.334 Å–1.338 Å shows frequencies of 1133.6 cm−1–1184.1 cm−1, suggesting non-dissociative O2 behaves as superoxide (O2–). To elucidate the atomistic evolution of oxidized surfaces in moist air or oxidizing solutions, we further explored interactions between H2O molecules and the most stable O2/H2O2 adsorption configurations. Results show that H2O dissociates into OH groups and H atoms, forming U–O and H–O bonds. This work provides new insights into O2/H2O2 adsorption morphologies on UO2 surfaces and their interactions with water at oxide interfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


