胎盘IGF1信号通路在产前PFAS暴露与不良出生结局之间的中介作用:来自系列中介模型的证据
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
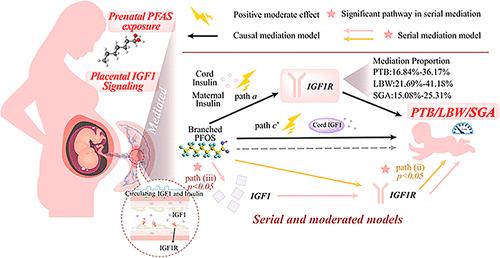
产前暴露于全氟烷基和多氟烷基物质(PFAS)与不良出生结局有关,但机制途径尚不清楚。我们测量了32名母亲的血清PFAS(包括它们的替代品和异构体),并量化了胎盘中胰岛素样生长因子1 (IGF1)、IGF1受体(IGF1R)和胰岛素受体(INSR)的mRNA水平,以及血清中IGF1和胰岛素的水平。我们应用简单、连续和调节的中介模型来研究胎盘IGF1信号作为pfas相关早产(PTB)、低出生体重(LBW)和小胎龄(SGA)的中介。简单中介显示,胎盘IGF1R介导了全氟辛烷磺酸(PFOS)异构体与PTB(比值比[or -总效应]:1.06-1.10)、LBW (or: 1.05-1.10)或SGA (or: 1.05-1.10)之间15.08%-41.18%的关联。串行中介鉴定出顺序通路:PFOS暴露与IGF1表达改变相关,其次是IGF1R变化,随后与PTB (or: 1.01-1.02)、LBW (or: 1.01-1.02)和SGA (or: 1.01-1.02)相关。有调节的中介强调血清IGF1和胰岛素作为这些关系的调节因子。分子对接表明支链PFOS优先结合IGF1R的配体结合域。本研究整合了先进的中介框架和分子证据,证明胎盘IGF1信号介导pfas相关的不良出生结果,阐明了发育毒性的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Mediation Role of the Placental IGF1 Signaling Pathway on Associations between Prenatal PFAS Exposure and Adverse Birth Outcomes: Evidence from Serial Mediation Models
Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) is associated with adverse birth outcomes, yet mechanistic pathways remain unclear. We measured 32 maternal serum PFAS (including their alternatives and isomers) and quantified placental mRNA levels of insulin-like growth factor 1 (IGF1), the IGF1 receptor (IGF1R), and the insulin receptor (INSR), alongside serum IGF1 and insulin levels, in 285 mother–infant pairs from the Maoming birth cohort. We applied simple, serial, and moderated mediation models to investigate placental IGF1 signaling as a mediator of PFAS-related preterm birth (PTB), low birth weight (LBW), and small-for-gestational-age (SGA). Simple mediation showed placental IGF1R mediated 15.08%–41.18% of associations between perfluorooctanesulfonate (PFOS) isomers and PTB (odds ratios [ORs-total effect]: 1.06–1.10), LBW (ORs: 1.05–1.10), or SGA (ORs: 1.05–1.10). Serial mediation identified a sequential pathway: PFOS exposure correlated with altered IGF1 expression, followed by IGF1R changes, and subsequent associations with PTB (ORs: 1.01–1.02), LBW (ORs: 1.01–1.02), and SGA (ORs: 1.01–1.02). Moderated mediation highlighted serum IGF1 and insulin as modifiers of these relationships. Molecular docking demonstrated preferential binding of branched PFOS to IGF1R’s ligand-binding domains. This study integrates advanced mediation frameworks and molecular evidence to demonstrate that placental IGF1 signaling mediates PFAS-related adverse birth outcomes, elucidating mechanisms of developmental toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


