冠状蛋白、cofilin和AIP1快速分解肌动蛋白丝的编排
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肌动蛋白丝(F-actin)网络的快速重塑对真核细胞的运动和形态发生至关重要。保守的肌动蛋白结合蛋白冠状蛋白、cofilin和肌动蛋白相互作用蛋白1 (AIP1)协同作用,促进f -肌动蛋白网络的快速分解,但其潜在机制尚不清楚。在这里,我们使用冷冻电子显微镜(cro - em),揭示了冠状蛋白、cofilin和AIP1的协同分子作用,导致肌动蛋白丝老化和断裂。我们发现冠状蛋白的协同结合变构性地促进了无机磷酸盐从f -肌动蛋白的释放,并诱导了丝的下扭,从而引发了丝的cofilin结合。然后,Cofilin通过限制链的协同结合机制将冠蛋白从丝中置换出来。由此产生的cofilactin作为AIP1的高亲和平台,它通过充当钳来破坏亚单位间的丝接触来诱导切断。在这种“分子挤压”机制中,AIP1而不是cofilin负责丝的切断。我们的工作重新定义了肌动蛋白动力学中关键分解因子的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
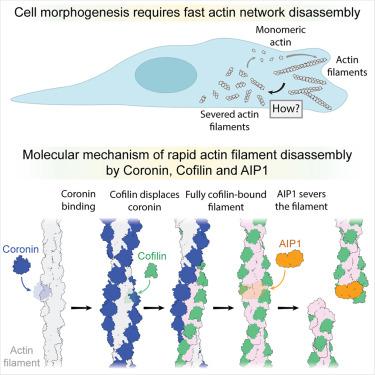
Choreography of rapid actin filament disassembly by coronin, cofilin, and AIP1
Rapid remodeling of actin filament (F-actin) networks is essential for the movement and morphogenesis of eukaryotic cells. The conserved actin-binding proteins coronin, cofilin, and actin-interacting protein 1 (AIP1) act in synergy to promote rapid F-actin network disassembly, but the underlying mechanisms have remained elusive. Here, using cryo-electron microscopy (cryo-EM), we uncover the concerted molecular actions of coronin, cofilin, and AIP1 that lead to actin filament aging and severing. We find that the cooperative binding of coronin allosterically promotes inorganic phosphate release from F-actin and induces filament undertwisting, thereby priming the filament for cofilin binding. Cofilin then displaces coronin from the filament via a strand-restricted cooperative binding mechanism. The resulting cofilactin serves as a high-affinity platform for AIP1, which induces severing by acting as a clamp that disrupts inter-subunit filament contacts. In this “molecular squeezing” mechanism, AIP1 and not cofilin is responsible for filament severing. Our work redefines the role of key disassembly factors in actin dynamics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


